2986
NMR and MRS studies of proteins delivered into cultured cells, towards MR analyses of proteins under physiological conditions
Airi Higashi1, Mitsuhiro Takeda1, Sosuke Yoshinaga1, and Hiroaki Terasawa1
1Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan
1Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan
Synopsis
We seek to study proteins
under physiological conditions, by delivering 13C-labeled proteins
into mouse brains and observing them by 13C MRS. We previously
observed 13C-labeled a-Synuclein (α-Syn)
embedded in an agarose gel by 13C MRS. To examine the feasibility of
MRS detection under more in vivo-like
conditions, we delivered α-Syn into cultured mammalian cells and observed the
cells by NMR, revealing that the delivered α-Syn
underwent intracellular interactions and N-terminal acetylation. For MRS
experiments with the cells, we constructed a system that perfuses the cells, to
prevent cell death over a longer time period.
Intrtoduction
Intracellular proteins undergo numerous effects under physiological conditions. α-Synuclein (α-Syn) is the major component of Lewy bodies, found in the brains of patients with dementia,1 and reportedly adopts a compact structure within cells.2 a-Syn presumably experiences molecular crowding, interactions with other molecules, and oligomerization/fibrillization under physiological conditions (Fig. 1). We sought to deliver 13C-labeled proteins into the mouse brain, to investigate them by 13C MRS. We previously detected 13C-labeled α-Syn embedded into an agarose gel by 13C MRS,3 which confirmed that protein signals can be detected by MRS. Recent NMR studies have observed stable isotope-labeled proteins delivered into cultured cells.2,4 We envisaged that cultured cells containing delivered target proteins can be used to test the feasibility of the MRS detection under more in vivo-like conditions. Therefore, we explored the delivery of isotope labeled proteins into cultured cells and the observations of the delivered proteins by NMR and MRS.Materials and Methods
HeLa S3 cells were treated with a pore-forming toxin, streptolysin O5 (20 ng/ml), and suspended in a solution containing 15N-labeled α-Syn (1 mM). After resealing by calcium chloride, 2.0 × 107 cells were suspended in 200 ml of Dulbecco's Modified Eagle's Medium, containing 25 mM HEPES (pH 7.5), 20% D2O, and 35% Redigrad (GE Healthcare), and then transferred to an NMR tube. The 1H-15N HSQC experiment was recorded at 10oC, using a 14.1 T NMR spectrometer (Bruker Biospin). The 13C-labeled α-Syn was embedded at 50 mM in Mebiol gel (Mebiol), and the gel was placed within a 2.5 ml syringe (Terumo). The MRS experiment was performed using a 7.0 Tesla Bruker BioSpec 70/20 scanner and a 13C mouse brain cryogenic coil (Bruker BioSpin).The 2.5 ml syringe was directly attached to the coil. Non-selective 13C MRS was recorded. MRS was also recorded with a syringe filled with only the Mebiol gel, which was used to subtract background signals. The cell viability was assessed by trypan blue staining. HeLa S3 cells were embedded in an alginate gel.Results
The 15N-labeled a-Syn was previously embedded in an agarose gel, but no significant changes were detected in the HSQC experiment, as compared to that in buffer (Fig. 2A).1 In contrast, when 15N-labeled α-Syn was delivered into HeLa S3 cells, the resulting spectrum was quite different from that in the buffer (Fig. 2B). Some peaks exhibited significant chemical shift changes and line-broadening, and were totally different from those obtained in agarose (Fig. 2C), presumably reflecting physiological effects that cannot be reproduced in the agarose gel. In our previous study, we found that the 13C signals of α-Syn in buffer and an agarose gel could be detected by MRS, with comparable quality to NMR (Fig. 3A, B, C). However, cells contain lipids and small molecules that produce 13C background signals, making it difficult to detect the α-Syn signals. We thus tested whether the α-Syn signals can be detected as a difference spectrum. α-Syn was embedded in the Mebiol gel, a copolymer of hydrophobic and hydrophilic polymers, as a proxy for biomolecules. We placed the gel in a 2.5 ml syringe and performed the 13C MRS experiment. After subtracting the spectrum of the Mebiol gel in the absence of α-Syn from that in the presence of α-Syn, the 13C signals of a-Syn were successfully detected even in the Mebiol gel (Fig. 3D). Next, we constructed a system for observing cultured cells by MRI. In this system, the cells are placed into a tube at a high density (~108 cells/ml), leading to nutrient depletion. Therefore, a bioreactor system,6 which perfuses the cells during the experiment, was constructed. In this system, the cells are embedded in an alginate gel and placed in a 2.5 ml syringe, with one end connected to a syringe pump and the other linked to a waste receptacle. To keep the cells in the syringe, the cells were entrapped within a gel, which was placed in the syringe (Fig. 4A). We constructed this system and confirmed that the viability of the cells was improved by the perfusion (Fig. 4B).Diiscussion
α-Syn delivery into cultured cells allows it undergo numerous physiological phenomena that cannot be reproduced in an agarose gel. We plan to perform MRS experiments on cells containing α-Syn, delivered using the perfusion system. In the in-cell NMR and MRS experiments, the cell density in the sample space is on the order of 108 cells/ml. Considering that the total number of neuronal cells in the human brain is on the order of 1011 cells and the volume of the brain is 1,500 ml, the cell density in the NMR and MRS experiments matches that of the brain regions. Thus, the MRS detection of proteins delivered into cultured cells serves as a milestone for achieving the in vivo detection by MRS.Acknowledgements
We thank Prof. Shimada and Dr. Nishida (Tokyo University) for advice regarding the in-cell NMR experiments.References
1. Iwai A et al. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron 1995; 14: 467-475 2. Rahman S et al. Towards understanding cellular structure biology: In-cell NMR. Biochim Biophys Acta Proteins Proteom. 2017;1865(5):547-557. 3. Saito K et al. NMR and MRS studies of the neurotoxic oligomer of alpha-Synuclein toward investigating its in vivo structure. Proc Intl Soc Mag Reson Med. 2017; 25: 5620 4. Theillet FX et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 2016; 530: 45-50 5. Ogino S et al. Observation of NMR signals from proteins introduced into living mammalian cells by reversible membrane permeabilization using a pore-forming toxin, streptolysin O. J Am Chem Soc. 2009;131(31):10834-10835. 6. Kubo S et al. A gel-encapsulated bioreactor system for NMR studies of protein-protein interactions in living mammalian cells. Angew Chem Int Ed Engl. 201;52(4):1208-11Figures
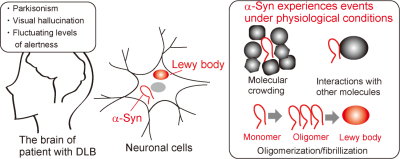
Figure 1. α-Synuclein is the major
component of the Lewy bodies, found in the brains of patients with dementia
with Lewy bodies (DLB). α-Syn experiences physiological
effects within the neuronal cells, such as molecular crowding, interactions
with other molecules, and oligomerization/fibrillization.
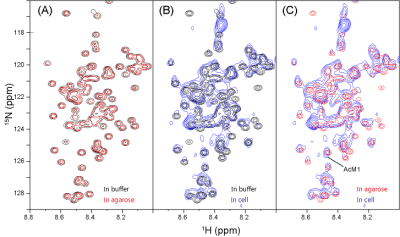
Figure 2. 1H-15N HSQC spectra of [U-15N]
α-Syn in phosphate buffer (black), 2% agarose (red),
and HeLa S3 cells (blue). For comparison, they are overlaid with a combination
of buffer/agarose (A), buffer/cells (B), and agarose/cells (C). These spectra were recorded using a 14.1 T
NMR spectrometer at 10°C.
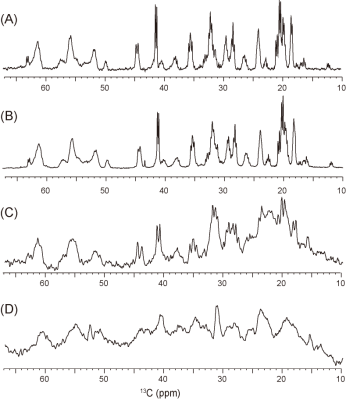
Figure 3. (A)
13C NMR spectrum of [U-13C/15N]-labeled a-Syn
in phosphate buffer. (B, C, and D) 13C
MRS spectra of [U-13C/15N]-labeled α-Syn
in phosphate buffer (B), 1% agarose (C), and Mebiol gel (D). Each experimental
time was approximately 4 h. The α-Syn concentration was 50 μM in
all samples.
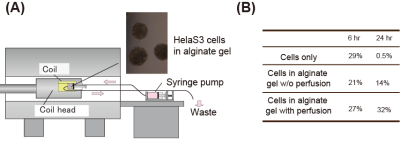
Figure 4. (A) Constructed system for observing cultured cells by MRI. The cells are embedded in the Mebiol gel and placed into a syringe. One end of the syringe is connected to a syringe pump and the other end is connected to a waste receptacle. (B) Comparison of cell viability between cell only, cell embedded in a gel without perfusion, and cell embedded with perfusion.