2985
Development of a 4-channel body coil for Deuterium Metabolic Imaging of the liver at 7T1Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
Deuterium Metabolic Imaging (DMI) is a new technique, which could potentially be used to study liver metabolism in vivo. The aim of this study was to develop a 4-channel 2H transmit/receive array for DMI of the liver at 7T, combined with 4 1H dipole elements for conventional anatomical MRI. Natural abundance DMI water and lipid maps measured on a phantom showed good correspondence with 1H Dixon water and lipid images. The multi-channel setup allowed us to pick up the natural abundance 2H water signal throughout the whole liver in a 3D DMI acquisition with a nominal resolution of 20x20x20 mm3.
Introduction
Deuterium (2H) metabolic imaging (DMI) is a new emerging technique to study metabolism in vivo and has been applied both in rats1,2 and in humans1. The disturbed metabolism of glucose in the diabetic liver could potentially be investigated non-invasively with DMI after oral intake of deuterated glucose. As the sensitivity of DMI scales supralinear with the magnetic field strength3, the application of DMI at ultra-high field leads to a significant gain in SNR, which can be employed to reduce scan time or to improve spatial resolution. In addition, DMI is characterized by relatively low radiofrequency (RF) power requirements, making the method extremely suited for application at ultra-high field, while 1H-MRI/MRS methods are typically challenging at high field due to much higher RF power demands. The aim of this study is to develop a 4-channel 2H transmit/receive array for DMI of the liver at 7T, combined with 4 1H dipole elements for conventional anatomical MRI.Methods
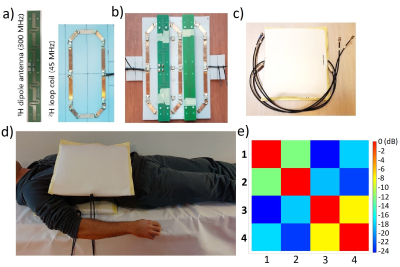
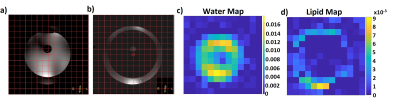
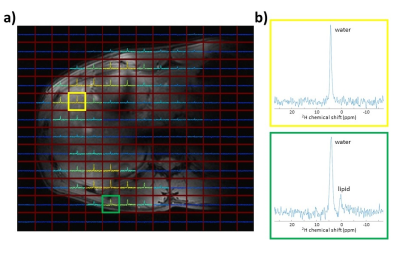
A DMI body coil setup was developed for a 7T whole-body MRI system (Philips Healthcare, Best, Netherlands), consisting of 4 2H transmit/receive loops. Two loops were incorporated in a posterior element and two other two loops in an anterior element (Figure 1). The loops within one element were geometrically decoupled. Each 2H transmit/receive loop was combined with a 1H dipole element for reference anatomical imaging and B0 shimming (Figure 1). All DMI measurements were performed with a pulse-acquire sequence using a 500 μs block pulse, followed by phase encoding gradients for 3D spatial encoding. Sensitivity profiles of the 2H coils were assessed individually on a body size phantom by driving each transmit coil consecutively. This was compared to an acquisition in which all 4 transmit coils were driven simultaneously (TR= 333 ms, FA= 90°, CSI matrix= 6 x 7 x 7, voxel size= 40 x 40 x 40 mm3, NSA= 4). Next, DMI was performed on a multi-compartment phantom, filled with water (inner compartment) and soybean oil (outer compartment). B1 and second order B0 shimming were applied before Dixon images were acquired, which were also used for CSI planning. A 3D DMI scan was performed (TR= 333 ms, FA= 90°, CSI matrix= 13 x 13 x 11, voxel size= 15 x 15 x 15 mm3, NSA= 4), from which water and lipid maps were generated by Lorentzian fitting and integrating the area under the respective peaks. For the in-vivo scan, coils were placed on the right lateral side of a healthy subject to maximize acquired signal from liver. The 3D DMI grid was planned on a Dixon scan and covered the whole liver. Acquisition was performed with elliptical k-space acquisition without respiratory gating (TR= 333 ms, CSI matrix= 12 x 14 x 13, voxel size= 20 x 20 x 20 mm3, NSA= 4, total acquisition time= 22 min). All 3D DMI data were constructed with Hamming filtering in the spatial domain, 10-Hz Gaussian apodization in the spectral domain and WSVD coil combination4 using the CSIgui toolbox5 in MATLAB.Results
Figure 2 shows sensitivity profiles of the 4 2H coils, as measured from the natural abundance HDO signal in a body size phantom, using single-channel transmit for each channel (left) and 4-channel transmit (right). The acquired signal patterns were similar for both cases, showing that there was no destructive interference between the effective B1+ fields of the 4 coils. Figure 3 displays 1H Dixon images (a,b) and DMI metabolite maps of the natural abundance 2H water and lipid signals (c,d) measured on a multi-compartment phantom filled with water and soybean oil, showing good correspondence in the localization of the water and lipid signals. The sensitivity of the developed setup was sufficient to pick up the natural abundance 2H signal from water in the liver (Figure 4). Signal could be detected throughout the whole liver; however, signal intensity dropped at further distances from the posterior and anterior coil elements.Discussion and Conclusion
This is the first application of a multi-channel body coil for DMI to our knowledge. Natural abundance DMI water and lipid maps measured on a phantom showed good correspondence with 1H Dixon water and lipid images. The developed multi-channel coil setup allowed us to pick up the natural abundance 2H water signal throughout the whole liver in a 3D DMI acquisition with a nominal resolution of 20 x 20 x 20 mm3. When the setup is combined with oral intake of deuterium labeled glucose or water in the next phase, it could enable us to study metabolic processes in the liver in more detail.Acknowledgements
This work was funded by a HTSM grant from NWO TTW (project number 17134).References
1.De Feyter HM, Behar KL, Corbin ZA, et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv. 2018;4(8):1-12. doi:10.1126/sciadv.aat7314
2.Lu M, Zhu XH, Zhang Y, Mateescu G, Chen W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2017;37(11):3518-3530. doi:10.1177/0271678X17706444
3.de Graaf RA, Hendriks AD, Klomp DW, et al. On the magnetic field dependence of deuterium metabolic imaging (DMI). Proc 27th Annu Meet ISMRM. 2019; #0492.
4.Rodgers CT, Robson MD. Receive array magnetic resonance spectroscopy: Whitened singular value decomposition (WSVD) gives optimal bayesian solution. Magn Reson Med. 2010. doi:10.1002/mrm.22230
5.CSIgui v2, http://ww.csigui.tk, visited on 25/09/2019.
Figures