2982
Quantitative Human Cardiac 39K MRI at 7T: What is feasible?1Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 3Center for Biomedical Imaging - Animal Imaging and Technology (CIBM-AIT), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 4Faculty of Medicine, University of Heidelberg, Heidelberg, Germany, 5Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 6MRI. TOOLS GmbH, Berlin, Germany, 7Institute of Radiology, University Hospital Erlangen, Erlangen, Germany, 8Institute of Medical Physics, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany
Synopsis
Monitoring the viability of myocardial tissue is of prognostic value after an ischemic event1-3. Ultrahigh fields (B0≥7T) facilitates cardiac 39K MRI. However, short relaxation times, low signal-to-noise ratio, and low spatial resolution render the quantitative determination of the myocardial tissue potassium concentration (mTPC) challenging. We analyze the feasibility of quantitative cardiac 39K MRI at 7T with simulations. For realistic noise conditions the mTPC can be determined with a deviation of 14% from the ground truth by applying a partial volume correction. Quantitative cardiac 39K MRI would further benefit e.g. from higher magnetic field strengths (B0>7T) or iterative reconstruction techniques.
Introduction
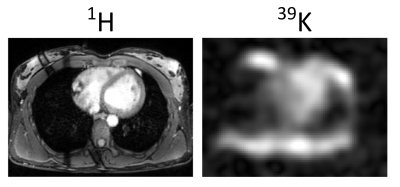
Potassium ions (K+) play an important role in biological processes and 39K MRI may provide information about tissue physiology and viability1,2. In acute and chronic myocardial infarction the tissue potassium concentration (TPC) is decreased3. As a supplement to commonly used ion concentration measurements of fluids e.g. from blood or urine, 39K MRI enables non-invasive quantification of the total tissue ion concentration. However, low spatial resolution, low signal-to-noise ratio (SNR) (SNR1H/SNR39K~458), extremely short transverse relaxation times (2-30ms4) as well as partial volume effects ,and respiratory and heart motion render quantitative 39K MRI challenging. In theory, Parrish et al.5 confirmed the feasibility of clinical cardiac 23Na MRI at B0 = 1.5T and indicated a requirement for an 8 fold higher SNR for 39K MRI. Responding to this request in vivo 39K MRI of human heart was recently demonstrated at B0 = 7T6 (Fig.1). Recognizing this progress, this work examines the feasibility of quantitative 39K MRI of the human heart using simulations to investigate the potential accuracy of myocardial TPC quantification at 7T.Methods
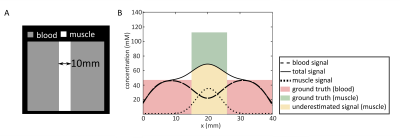
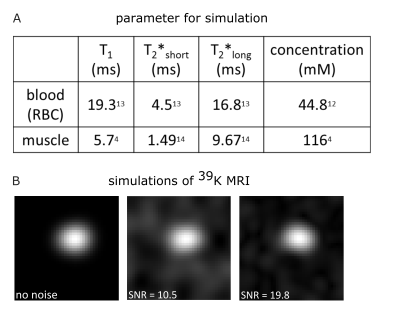
Cardiac 39K MRI simulations7 were executed using binary masks of blood volume and myocardium based on in vivo 1H acquisitions at 3T (navigator-gated, ECG-triggered Flash sequence8, (∆x = 2mm)3 interpolated to (∆x = 1mm)3 , TE = 1.3ms, TR = 3ms, α = 20°, tacquisition(ACQ) = (6-10)min). For each mask, k-space data were regridded to a radial acquisition trajectory and scaled to the approximated potassium concentration (Fig.3). Relaxation effects were considered and Gaussian noise was added. Due to the approximately 40-fold higher intracellular potassium concentration compared to blood serum, relaxation of extracellular potassium was neglected and relaxation properties of potassium in red blood cells were assumed for blood. For the myocardium, relaxation properties and TPC of calf muscle were assumed. Hence, bi-exponential signal decays were applied for myocardium and blood. Simulation parameters were similar to those of recently published in vivo measurements6 (Fig.1): (∆x = 14.6mm)3, TE = 0.7ms, TR = 30ms, tACQ = 30min, tpulse = 1ms.39K simulations were reconstructed with a non-uniform Fast Fourier transform9 using a Hanning filter. Heart and respiratory motion as well as field effects (B0, B1) were not considered in the simulations. Due to the large voxel size and small thickness of the myocardium, partial volume effects occur (Fig.2). A binary mask-based partial volume correction10 (PVC) was applied to the 39K simulations before the data were corrected for relaxation effects and normalized to blood.
To validate the simulation set-up, cardiac 39K MRI data were simulated both without noise and with the SNR (SNR~10) achieved in previous in vivo measurements6. In addition, a simulation with twice the SNR was performed to evaluate the effect of potential future measurements at higher magnetic field strength. Simulations with random noise were repeated five times to analyze the influence of the resulting SNR on the determined TPC.
Results
Fig.3 shows the simulated cardiac 39K images with and without noise. The myocardium (mean width in 1H MRI: 9mm) cannot be directly resolved in the MR image. Due to partial volume effects signal spills into adjacent regions.Without PVC, the determined TPC is underestimated by (56±1) % (Fig.4). For ideal data (simulation without noise) the PVC can recover the input TPC of the myocardial mask. For simulations with noise the resulting mean TPC of five simulations is underestimated by 14 % and 3 % for a SNR = (10±1.9) and SNR = (20±0.7), respectively, and the variation of the myocardial TPC (∆TPCmyocardial) of the five simulations decreased with higher SNR: ∆TPCmyocardial,SNR~10 = 14mM and ∆TPCmyocardial,SNR~20 = 5mM.
Discussion and Conclusion
The determination of myocardial TPC was investigated based on simulations. Due to the high intracellular TPC compared to the relatively low potassium concentration in blood, the applied PVC exhibits a large influence and therefore represents an essential correction for the determination of the myocardial TPC when large voxel sizes are investigated.The accuracy of the applied PVC relies on the accuracy of the myocardial relaxation times which were approximated with those of calf muscle. With the true relaxation times the impact of the PVC on the resulting TPC might slightly differ.
Compared to quantitative cardiac 23Na MRI, which is ready for clinical studies11, cardiac 39K MRI at 7T is feasible, but the reliability of the quantification is less reliable. Despite approaching the theoretically required SNR5 proposed for 39K MRI, the resulting myocardial TPC of five independent simulations with the actual SNR at 7T shows a larger variation and a higher underestimation from the input ground truth than for simulations with twice the SNR.
Thus, for realistic noise conditions, the potassium concentration of the myocardial tissue can be estimated, but with a deviation of 14% from the ground truth. Quantitative cardiac 39K MRI would thus highly benefit from higher SNR e.g. from a higher magnetic field strength (B0>7T), longer measurement times, optimized sequence protocols, adapted RF coils or iterative reconstruction techniques15.
Acknowledgements
This work was supported by iMed – the Helmholtz Initiative on Personalized Medicine.References
1. Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev 1999; 79(3): 917-1017.
2. Haddy FJ et al. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006; 290(3): R546-552.
3. Fieno DS et al. Physiological basis for potassium (39K) magnetic resonance imaging of the heart. Circ Res. 1999; 84(8): 913-920.
4. Umathum R et al. In vivo 39K MR imaging of human muscle and brain. Radiology. 2013; 269(2): 569-576.
5. Parrish TB et al. Theoretical basis for sodium and potassium MRI of the human heart at 1.5 T. Magn Reson Med. 1997; 38(4): 653-661.
6. Wenz D et al. In vivo potassium MRI of the human heart. Magn Reson Med. 2019
7. Lommen JM et al. Realistic simulation of 23Na brain data: Understanding the influence of acquisition parameters on the accuracy of 23Na concentration measurement. Proc Intl Soc Mag Reson Med. 25 2017; 5628.
8. Bi X et al. Whole‐heart coronary magnetic resonance angiography at 3 Tesla in 5 minutes with slow infusion of Gd‐BOPTA, a high‐relaxivity clinical contrast agent. Magn Reson Med. 2007; 58: 1–7.
9. Fessler JA et al. Nonuniform fast Fourier transforms using min-max interpolation. IEEE Trans Signal Process. 2003; 51(2): 560-574.
10. Niesporek SC et al. Partial volume correction for in vivo 23Na-MRI data of the human brain. NeuroImage. 2015; 112: 353-363.
11. Lott J et al. Corrections of myocardial tissue sodium concentration measurements in human cardiac 23Na MRI at 7 Tesla. Magn Reson Med. 2019; 82.1: 159-173.
12. Hald PM. Notes on the determination and distribution of sodium and potassium in cells and serum of normal human blood. J Biol Chem. 1946; 163: 429-434.
13. Shinar H et al.Single and Multiple Quantum Nmr Relaxation-Times of Sodium and Potassium in Red-Blood-Cells. Israel Journal of Chemistry. 1992; 32(2-3): 299-304.
14. Roesler MB et al. In vivo observation of quadrupolar splitting in (39)K magnetic resonance spectroscopy of human muscle tissue. NMR Biomed. 2016; 29(4): 451-457.
15. Behl NG et al. Three-dimensional dictionary-learning reconstruction of (23)Na MRI data. Magn Reson Med 2016; 75(4):1605-1616.
Figures