2978
Sodium TQ signal cancelation due to B0 inhomogeneities despite the use of a refocusing pulse
Dennis Kleimaier1 and Lothar R. Schad1
1Computer Assisted Clinical Medicine, Heidelberg University, Mannheim, Germany
1Computer Assisted Clinical Medicine, Heidelberg University, Mannheim, Germany
Synopsis
The sodium TQ signal is sensitive to B0 inhomogeneities leading to TQ signal cancelation. In this study, we evaluated three different shim routines to assess the robustness to B0 inhomogeneities and the reproducibility of the sodium TQ signal. Using an optimized shim routine, the TQ signal dependence on potassium concentration was investigated. Severe B0 inhomogeneities caused a reduction in the TQ signal, despite using a refocusing pulse, without affecting the transversal relaxation times. To achieve reproducible TQ measurements, the FWHM of the sodium SQ signal must be specified. Addition of different amounts KCl caused a reduction in the TQ signal.
Introduction
The interaction of sodium ions with negatively charged groups of proteins create a sodium triple-quantum (TQ) signal. The sodium TQ signal has an intracellular weighting1-3 and can be used for the quantification of cellular responses4. The TQ signal is sensitive to B1+ and B0 inhomogeneities. A volume coil minimizes B1+ deviations while B0 inhomogeneities can be mitigated by a refocusing pulse or an extended phase cycle5,6. However, the robustness to B0 inhomogeneities of the sodium TQ signal when using a refocusing pulse still has to be investigated.In this study, we used the TQ time proportional phase incrementation (TQTPPI) sequence to simultaneously measure the sodium TQ and single-quantum (SQ) signal at distinct offset frequencies7,8. Three different shim routines, leading to different B0 inhomogeneitites, were used to assess the robustness of the TQ signal against B0 inhomogeneities when using a refocusing pulse. By repeating the shim process, the reproducibility of the TQ measurement was evaluated. The optimized shim routine was used to verify the sodium TQ signal dependence on the potassium concentration which was indicated by Schepkin et al.8. Therefore, the TQ signal of the protein bovine serum albumin (BSA) in various KCl concentrations was measured.
Material and Methods
A 9.4T preclinical Bruker MRI was used for data acquisition. A 1H/23Na Bruker volume coil was used to reduce B1+ inhomogeneities.A TQTPPI sequence (TR=300ms, 720 time steps) with a refocusing pulse placed between the first two 90° pulses was used to mitigate B0 inhomogeneities that cause TQ signal cancellation (Fig.1a). The TQTPPI FID was nonlinearly fitted according to8.
A small phantom (diameter=2.7cm, height=3cm) with 5% w/w agarose and 154mM NaCl was used to investigate the robustness of the TQ signal against B0 inhomogeneities (Fig.1b). The small phantom size was chosen to cover only the homogeneous excitation region of the volume coil. This minimizes flip angle deviations. Three different shim routines were used:
- 23Na global first and second order Bruker shim routine
- 1H global first and second order Bruker shim routine
- Acquisition of a 1H B0 map and subsequently application of a first and second order global Bruker shim routine (1H map shim)
The optimal shim routine was used to verify the dependence of the sodium TQ signal on the potassium concentration. To do so, 8ml of 10% w/v BSA in 154mM NaCl with different concentrations of [0, 10, 25, 50, 75, 100, 125, 145]mM KCl were prepared.
Results and Discussion
Severe B0 inhomogeneities led to a reduction of the TQ signal and an increase of the noise in the TQTPPI spectrum (Fig.2a). For all measurements, an increase of the ATQ/ASQ value for smaller FWHM was observed (Fig.2b). Only for the 1H map shim routine the ATQ/ASQ value was reproducible for each shim process repetition. The largest variations in ATQ/ASQ were observed for the 1H global shim routine (Fig.3a). However, the transversal relaxation times were not influenced by a different severity of B0 inhomogeneities (Fig.3b,c). The mean values of T2S, T2F and ASQS/ASQ agreed within the 95% confidence intervals for all three shim routines (Tab.1). ATQ/ASQ agreed within the 95% confidence interval only for the 23Na and 1H global shim routine. These results showed that, despite the use of a refocusing pulse, a good shim condition is required to achieve a reproducible maximum TQ signal amplitude. The optimal shim routine may differ for different shaped phantoms. Therefore, the optimal shim routine must always be evaluated. In order to increase the TQ reproducibility of phantom measurements from different studies, the sodium SQ FWHM must be specified. On the other hand, the robustness against B0 inhomogeneities can be increased by combining a refocusing pulse with an extended phase cycle for B0 correction5,6.For all BSA measurements, the FWHM was 55.4±2.9Hz. Up to 25mM KCl the ATQ/ASQ ratio did not change (Fig.4b). For higher concentrations of KCl, a decrease in ATQ/ASQ was observed (Fig.4b). The addition of 145mM KCl reduced the ATQ/ASQ value to 79.1±3.0% compared to the ATQ/ASQ value from 0mM KCl. In addition, T2S increased from 35.7±0.3ms to 37.2±0.3ms while T2F increased from 28.0±0.4ms to 29.4±0.5ms (Fig.4a). Our results demonstrate a sodium TQ signal dependence on the potassium concentration.
In agreement with our results, Schepkin et al.8 observed a reduction of the ATQ/ASQ value to 85.8±3.4% and 89.2±3.1% for 5% and 7.5% agarose in 154mM NaCl compared to 5% and 7.5% agarose in 154mM NaCl and 154mM KCl, respectively. Similar to our measurements, an increase of both transversal relaxation times was observed8.
Conclusion
The evaluation of the optimal shim routine revealed a strong dependence of the sodium TQ signal on the B0 inhomogeneities despite the use of a refocusing pulse. This indicates the importance to specify the sodium SQ FWHM to allow for reproducible TQ measurements. By using the optimal shim routine, a sodium TQ signal dependence on the potassium concentration was demonstrated.Acknowledgements
No acknowledgement found.References
- Knubovets T, Shinar H, Navon G. Quantification of the contribution of extracellular sodium to Na-23 multiple-quantum-filtered NMR spectra of suspensions of human red blood cells. J Magn Reson 1998;131(1):92-96.
- Seshan V, Sherry AD, Bansal N. Evaluation of triple quantum-filtered 23Na NMR spectroscopy in the in situ rat liver. Magnetic resonance in medicine. 1997;38(5):821-827.
- Winter PM, Bansal N. Triple-quantum-filtered (23)Na NMR spectroscopy of subcutaneously implanted 9l gliosarcoma in the rat in the presence of TmDOTP(5-1). Journal of magnetic resonance. 2001;152(1):70-78.
- Schepkin VD, Choy IO, Budinger TF. Sodium alterations in isolated rat heart during cardioplegic arrest. Journal of applied physiology. 1996;81(6):2696-2702.
- Fleysher L, Oesingmann N, Inglese M. B(0) inhomogeneity-insensitive triple-quantum-filtered sodium imaging using a 12-step phase-cycling scheme. NMR in biomedicine. 2010;23(10):1191-1198.
- Tanase C, Boada FE. Triple-quantum-filtered imaging of sodium in presence of B(0) inhomogeneities. Journal of magnetic resonance. 2005;174(2):270-278.
- Hoesl MAU, Kleimaier D, Hu R, et al. (23) Na Triple-quantum signal of in vitro human liver cells, liposomes, and nanoparticles: Cell viability assessment vs. separation of intra- and extracellular signal. Journal of magnetic resonance imaging : JMRI. 2019;50(2):435-444.
- Schepkin VD, Neubauer A, Nagel AM, Budinger TF. Comparison of potassium and sodium binding in vivo and in agarose samples using TQTPPI pulse sequence. Journal of magnetic resonance. 2017;277:162-168.
Figures
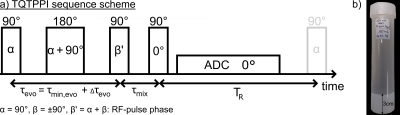
Fig.1: a)
The TQTPPI sequence contains a 180° refocusing pulse between the first two 90°
pulses to mitigate B0 inhomogeneities. b) The 5% w/w agarose phantom
is shown. The diameter is 2.7cm and the height is 3cm.
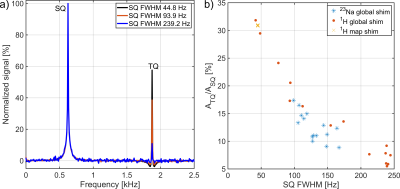
Fig.2: a)
Severe B0 inhomogeneities led to a decrease in the TQ signal
amplitude and an increase in the noise of the TQTPPI spectrum. b) The different
repetitions of the 23Na and 1H global shim routine resulted
in a spread of ATQ/ASQ values due to differences in the
sodium SQ FWHM. This showed a significant dependence of the TQ signal on B0
inhomogeneities despite the use of a refocusing pulse.
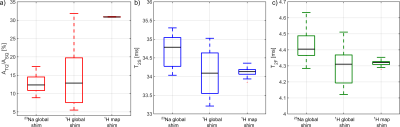
Fig.3: a)
To achieve a reproducible TQ signal, a good shim condition is required despite
the use of a refocusing pulse. b), c) Both transversal relaxation times were
not affected by different B0 inhomogeneities.
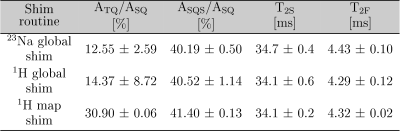
Tab.1: a)
ASQS/ASQ, T2S and T2F agree within
the 95% confidence interval for all three shim routines. The ATQ/ASQ
value for the 1H map shim routine is significantly higher than the
value from the other two shim routines.
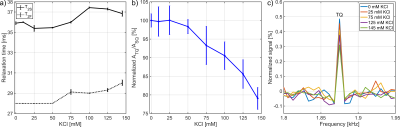
Fig.4: a)
Both relaxation times increased for increasing KCl concentration. b) The ATQ/ASQ
value decreased for increasing KCl concentration. For 145mM KCl, the ATQ/ASQ
value decreased to 79.1±3.0% compared to 0mM KCl. c) Zoomed TQTPPI spectrum that
only shows the TQ signal. For increasing KCl concentration, the TQ signal
decreased.