2977
Corrections of cardiac 23Na-MRI measurements in mice with an in-house build surface coil at 7T1Comprehensive Heart Failure Center (CHFC), Department of Internal Medicine I, University Hospital Wuerzburg, Wuerzburg, Germany, 2Comprehensive Heart Failure Center (CHFC), Chair of Cellular and Molecular Imaging, University Hospital Wuerzburg, Wuerzburg, Germany
Synopsis
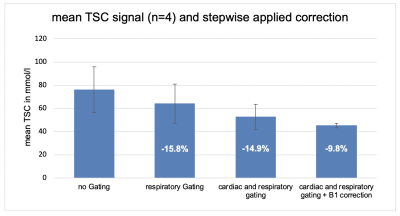
With higher field strength and, thus, better SNR available, 23Na-MRI is becoming increasingly popular in (pre-) clinical studies. Improving accuracy and repeatability will help to translate 23Na-MRI into routine. Therefore, we investigated the effect of respiratory and cardiac gating, as well as the influence of B1-field correction on cardiac total sodium quantification with a 2D radial UTE sequence in mice using a surface coil at 7T. We found that ungated and uncorrected measurements overestimate cardiac sodium concentration. Combined respiratory and cardiac gating and B1-field correction significantly decreased the measured cardiac tissue sodium concentration (-40.6%).
Introduction
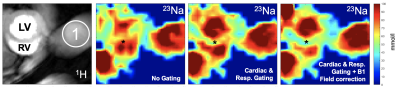
Sodium is regarded as a marker of ion homeostasis and driver of biological processes.1,2 With higher field strength available there is a renaissance of sodium MRI as it allows the non-invasive quantification of tissue sodium concentration (TSC). In order to improve accuracy of TSC measurements, previous studies investigated the effects of various factors on the sodium signal using birdcage coils.3 However, with regard to the physical sensitivity, surface coils would be prefereable. Therefore, we investigated the influence of respiratory and cardiac gating, as well as B1-field distribution on the cardiac sodium signal using an in-house-built dual-tune 23Na/1H surface coil (Setup, see Figure 1). The effects of each gating setup and B1-field correction were compared to baseline uncorrected data to demonstrate the scope of TSC signal changes.Methods
Mouse experiments were performed in accordance with local laws and official permit (TVA RUF-55.2.2-2532-2-735-16) of the responsible authorities at a 7T Bruker small animal scanner (PharmaScan 70/16 Bruker BioSpin GmbH, Ettlingen, Germany). We used an in-house build dual-tuned 23Na/1H spiral surface coil, which was inspired by the design from Wetterling et al.4 To monitor ECG and respiratory motion, and also for prospective triggering, a Model 1030 animal monitoring and gating system (SA Instruments Inc., Stony Brook, NY) was used. For imaging, a short axis view at the midventricular level was selected in 1H-MRI. Subsequently, a 2D radial UTE sequence was used to assess the sodium signal (TE: 0.35 ms; TR: 100 ms, Treadout: 0.365 ms, AQbandwidth: 65 kHz, Flip angle: 90°, 150 projections, FOV 35x35 mm², Slice thickness 4 mm, Acquisition-Matrix 48x48 resulting in a nominal resolution of 1.96 mm³, approx. 2 µl/Voxel, 70 Averages, acquisition time TAQ: ~26 min). Three measurements were performed for each mouse (n=4). One without gating, one with respiratory gating, and one with combined cardiac and respiratory gating to image the fully relaxed heart (diastolic state). For B1-field correction, B1 maps were acquired using the double angle method (DAM).5 DAM Maps were acquired using a gradient echo sequence with TE 0.8 ms, TR 200 ms, Treadout: 0.365 ms, FA 45° and 90°, 70 Averages, same resolution as the 2D-UTE, TAQ ~14 min for both). An external vial with known sodium concentration was used as a reference for total sodium quantification. B1-field correction and sodium quantification were done using a custom MATLAB script (Matlab2015b, MathWorks, Massachusetts, United States).Results
Quantitative sodium imaging showed a sodium signal of 76.1±19.6 mmol/l (mean±SD) without gating or B1-field correction, 64.0±16.8 with respiratory gating and 52.7±10.9 with cardiac and respiratory gating to diastolic state. Applying B1-field correction further reduced the signal to 45.2±1.8 mmol/l. Hence, respiratory gating reduced the measured cardiac TSC by 15.8 %, combining cardiac and respiratory gating additionally reduced the TSC by 14.9% and B1-field correction added another 9.8% of signal reduction. Thus, uncorrected data overestimates cardiac TSC and the applied correction methods reduced the signal in total by 40.6%. (Figure 2)Discussion
Our results demonstrate the effect of different gating set-ups and the applied B1-field correction on the sodium signal measured with a surface coil at 7T. In previous studies with birdcage resonators, B1-field correction only reduced the signal by 3%. In contrast when using surface coils B1-field correction has a major influence on the TSC quantification reducing the signal by 9.8% alone. A greater impact on data acquisition showed the proper gating on the diastolic phase of the heart (combined prospective respiratory and cardiac gating) with a signal reduction of -30.7%, assumedly by reducing partial volume and motion related overlapping effects. Calculating the theoretical cardiac TSC of mice using a formula proposed by Bottomly6 and values from literature, estimated TSC is 43 mmol/l; thus our results after correction (45.2±1.8 mmol/l) are in good agreement.Conclusion
With higher field strength available, sodium MRI is becoming feasible and more commonly used in preclinical and clinical studies. Our results demonstrate the importance of proper gating and B1-field correction to increase the accuracy of TSC measurements when using surface coils. Higher accuracy and repeatability will help to establish sodium MRI in (pre-) clinical routine.Acknowledgements
Financial support from the German Ministry of Education and Research (BMBF grant #01EO1504 ) is appreciated.References
1. Boada, F. E. et al. Loss of Cell Ion Homeostasis and Cell Viability in the Brain: What Sodium MRI Can Tell Us. in Current Topics in Developmental Biology vol. 70 77–101 (Elsevier, 2005).
2. Madelin, G. & Regatte, R. R. Biomedical applications of sodium MRI in vivo: Biomedical Applications of Sodium MRI. J. Magn. Reson. Imaging 38, 511–529 (2013).
3. Lott, J. et al. Corrections of myocardial tissue sodium concentration measurements in human cardiac 23 Na MRI at 7 Tesla. Magn. Reson. Med. 82, 159–173 (2019).
4. Wetterling, F. et al. The design of a double-tuned two-port surface resonator and its application to in vivo Hydrogen- and Sodium-MRI. J. Magn. Reson. 217, 10–18 (2012).
5. Insko, E. K. & Bolinger, L. Mapping of the Radiofrequency Field. J. Magn. Reson. 103, 82–85 (1993).
6. Bottomley, P. A. Sodium MRI in human heart: a review. NMR Biomed. 29, 187–196 (2016).
Figures