2970
A Perfusate Comparison for Ex-Vivo Lung Preservation: Using 31P MRS to Assess Cold Perfadex and Modified Krebs–Henseleit Buffer1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Surgery, University of Pennsylvania, Philadelphia, PA, United States, 3Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 4Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States, 5Physiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Ex-vivo lung perfusion (EVLP) is a procedure used in clinical care to support lung viability during transplantation. Here, we used magnetic resonance spectroscopy (MRS) to monitor and compare the energy status of ex-vivo rat lungs flushed and stored in cold Perfadex or perfused with a modified warm Krebs-Henseleit buffer. The results demonstrate decreasing energy status over the course of the 3hr experiment under both conditions, with a slightly lower rate of decline in lung viability using cold Perfadex.
Introduction
A significant shortage of available donor lungs presents a hurdle to the wide-spread use of lung transplantation to treat end-stage lung disease. Lungs sourced after donor brain death may alleviate this supply shortage, but organ management and preservation strategies are needed due to the increased risk of ischemia-reperfusion injury, primary graft dysfunction, and post-transplant complications in such lungs [1]. Ex-vivo lung perfusion is one such preservation strategy, supporting lung tissue oxygenation, metabolic activity, and energy status. EVLP also provides a mechanism for monitoring lung viability with metabolic biomarkers, as well as the possibility for real-time intervention. The current standard of care (SOC) excises the donor lung, flushes it with cold Perfadex, and stores it at 4-8oC [2]. In this study, a rat model was used to compare lung graft viability after static cold preservation using cold SOC vs. EVLP with a warm modified Krebs–Henseleit buffer (perfusate) by monitoring lung energy status via magnetic resonance spectroscopy of metabolites.Materials and Methods
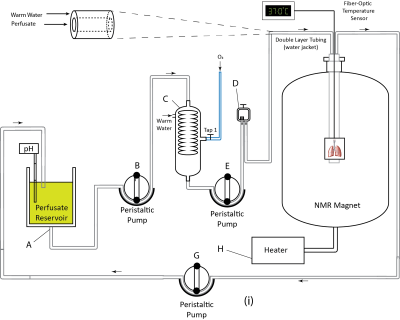
Sprague Dawley (n=6) rats were used for this study. Animals were mechanically ventilated for 10 minutes (VT = 3.5 ml, f = 38 min-1, FiO2 = 1.0, PEEP = 0 cmH2O) and lungs were rapidly excised and placed in a 20 mm NMR tube before being transferred to the MRI machine (AVANCE III 9.4T, Bruker Inc.). Lungs were perfused for 3 hours through the pulmonary artery at a 10 ml/min flow rate with 500 ml of a modified Krebs-Henseleit buffer (119 mM NaCl, 25 mM NaHCO3, 1.3 mM CaCl2, 1.2 mM MgSO4, 4.7 mM KCl, 10 mM glucose, 2 mM lactate, 0.2 mM pyruvate, and 3% (w/v) fatty-acid-free bovine serum albumin). This setup is shown in Figure 1. The perfusate was passed through an oxygenating column under a constant flow of oxygen and warmed via passage through heated water-jacketed tubing to maintain a temperature of ~37oC in the NMR tube. pH was periodically adjusted to ~7.4 by adding 1.2 N HCl or NaOH to the perfusate reservoir. After 1 hour of perfusion, three lungs were removed from the magnet, flushed with 20ml of 4oC Perfadex+ [XVIVO Perfusion], and stored in 4oC Perfadex+ for 1 hour (SOC model), after which normal perfusion was resumed and lungs were returned to the MRI machine. The remaining lungs (n=3) remained in the spectrometer throughout the entire study (perfusate model). 31P spectra were obtained using a 25-mm dual-tuned (1H/31P) coil (Bruker Inc.) with the following acquisition parameters: TR=1 s, FA=60o, SW=8 kHz, NP=2048, NA=1024, with a total acquisition time of ~17 minutes. Data were processed using custom routines in MATLAB 2018a and RStudio 1.2.13.Results and Discussion
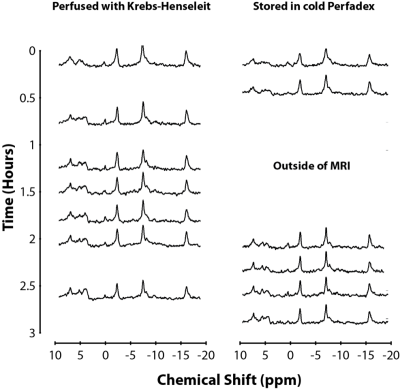
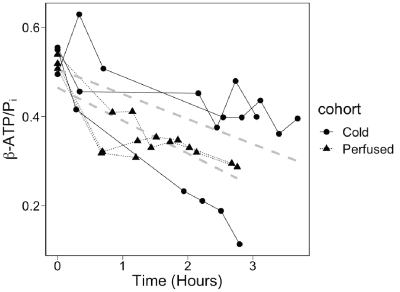
As lung energy status and mitochondrial function decline, a concomitant decline in ATP signal can also be observed alongside an increase in Pi signal, resulting in a lower β-ATP/Pi metabolite ratio [3]. Results shown in Figure 2 demonstrate that both SOC and perfusate models exhibited a similar decline in ATP signal coupled with an increase in Pi. Figure 3 shows that lungs treated with SOC exhibited higher β-ATP/Pi levels and a slightly lower rate of decline compared to the perfusate model over the course of the experiment. The fact that SOC lungs had higher β-ATP/Pi ratios both at the start and throughout the experiment suggests that the SOC lungs were healthier than the perfusate lungs, possibly independent of the treatment. Based on the SOC lungs’ slightly lower rate of decline in energy status vs perfusate lungs, the preservation benefit may only be minor. Additionally, one observation in the SOC model which lowered the overall observed preservation ability may be an outlier: one SOC lung’s initial β-ATP/Pi ratio was similar to the other 2 SOC organs, indicating similar initial lung energy status; however, this ratio rapidly declined soon after, suggesting a deterioration in the lung, possibly caused by an O2 embolism. When including this lung, the SOC and perfusate models’ rates of decline were not statistically different.Conclusion
While the SOC maintained the lung’s energy status better than the warm modified Krebs-Henseleit buffer, the difference was not statistically significant, warranting further testing of both models as well as the examination of additional metrics. Several studies have demonstrated the potential utility of using hyperpolarized 13C pyruvate to evaluate lung health [4]. Future studies will therefore use a combination of 31P and 13C MRS in order to obtain a comprehensive characterization of lung bioenergetics by differentiating the respective contributions of phosphorylation and glycolysis to the preservation of lung energy production. This combined imaging technique could potentially provide a powerful tool for assessing various static preservation or perfusion strategies to improve lung viability for transplantation.Acknowledgements
No acknowledgement found.References
[1] R. Watts, et al. Journal of Transplantation. 2013; 2013: 521369
[2] L. Munshi, et al. Lancet. 2013; 1(4): 318-328
[3] H. Shaghaghi, et al. Free Radical Biology and Medicine. 2015; 89: 62-71
[4] H. Shaghaghi, et al. NMR in Biomedicine. 2013; 27: 939-947