2968
Feasibility of 31P MR Imaging at 7T1Center of Image Sciences, UMC Utrecht, Utrecht, Netherlands
Synopsis
Typical 31P CSI acquisitions have long scan times with a course resolution. Recent technical developments have incorporated a transmit body coil and receiver array for 31P in 7T systems. This opens up the possibility to acquire 31P data similar to conventional 1H imaging. In this study, we investigated the feasibility of 31P gradient echo MRI to measure the 31P distribution in human muscle.
Introduction
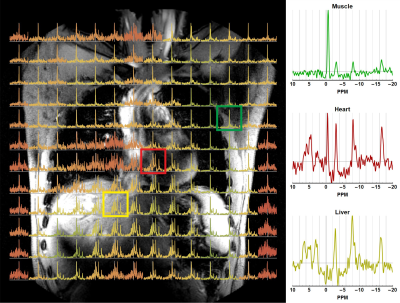
Magnetic resonance spectroscopy and spectroscopic imaging is used to obtain information on 31P metabolites related to, e.g., energy or cell membrane metabolism. Despite moving to high field strengths, such as 7T or higher, the resolution of CSI acquisition remains fairly course with long scan times due to the low natural abundance of 31P in the body.Recent developments incorporated a transmit body coil tuned for 31P, 121MHZ, and a 16 channel 31P receiver array into the system1,2,3, to facilitate homogeneous B1+ fields combined with sensitive receiver arrays. These developments allow to increase spatial coverage and resolution with high SNR in reasonable acquisition times for whole body CSI. As importantly, this opens up the possibility to perform 31P MRI at 7T and the use of all MRI methods available for 1H considering distinct metabolic profiles with substantial variances in spatial domain (figure 1).
In this study, we investigated the feasibility to obtain 31P gradient echo MRI images to measure the 31P distribution in muscle, and compared this to conventional 31P CSI.
Methods
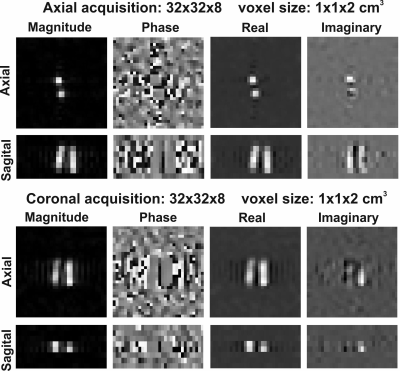
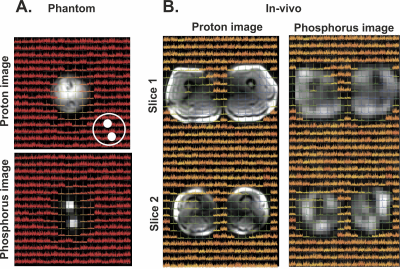
All experiments were performed at a whole body 7T MR system (Philips Healthcare, Best, NL) equipped with a home-built integrated quadrature transmit body coil and a 16-channel local receiver array, both tuned for 31P (121 MHz). Proton information was obtained using an 8-channel Tx/Rx antenna array embedded in the receiver array.A set of three 31P experiments was performed on a phantom consisting of 2 tubes (2cm diameter) containing a 0.1M phosphate solution embedded in 1H containing tube, as well as on the thigh muscles of a healthy volunteer. The experiments comprised a 3D CSI scan, a 2D multi-slice gradient echo scan, and a 2D multi-slice multi-echo gradient scan, detailed parameters are shown in the table in figure 2. For both cases, a 1H gradient echo scan was performed for localization purposes.
Data reconstruction and processing was performed offline using the open source package QMRI Tools4. For both the imaging and the CSI data the raw 16 channel data was reconstructed using SENSE reconstruction where the coil sensitivity maps were estimated by dividing the individual coil data by the sum of squares addition of all coils.
Results
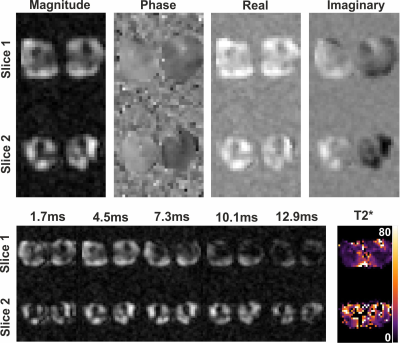
Figure 3 shows the gradient echo image of the phantom experiment, where both tubes were clearly visible.Figure 4 shows the transversal multi-echo gradient echo 31P images of the in-vivo experiment where both legs are clearly visible. In the top row displays the results of the multi-slice gradient echo image. The bottom row displays the magnitude images of the multi-echo acquisition, as well as the fitted T2* map. Fitted T2* values were in the order of 20ms. Signal voids in the leg are the bones, where no 31P signal is expected.
Figure 5 shows the CSI spectra overlaid on the 1H as well as the 31P MRI acquisition, both for the phantom and in-vivo experiments. A good correlation was found between the 1H image, the 31P image and the CSI data, where the 31P has a better spatial localization compared to the CSI data.
Discussion and conclusion
This study shows that 31P MR imaging is feasible, using a transmit body coil and a receiver array at 7T. Currently, 31P MRI does not quantify the metabolites to the extent as MRS does but is can allow for better spatial localization. The feasibility of 31P MRI will allow the use of all quantitative 1H MRI methods. Therefore, using methods such as Dixon can potentially allow for metabolite quantification.Acknowledgements
No acknowledgement found.References
1. Löring et al., Whole‐body radiofrequency coil for 31P MRSI at 7 T, https://doi.org/10.1002/nbm.3517
2. Valkovič et al., Low SAR 31P (multi‐echo) spectroscopic imaging using an integrated whole‐body transmit coil at 7T, https://doi.org/10.1371/journal.pone.0187153
3. van Houtum et al., Low SAR 31P (multi‐echo) spectroscopic imaging using an integrated whole‐body transmit coil at 7T, https://doi.org/10.1002/nbm.4178
4. Froeling, QMRTools: a Mathematica toolbox for quantitative MRIanalysis, http://doi.org/10.21105/joss.01204
Figures