2956
Investigation of lung cancer early detection with serum MRS biomarkers
Leo Ling Cheng1, Tjada A. Schult1,2, Mara J. Lauer1,3, Lindsey A. Vandergrift1, Mari A. Mino-Kenudson4, and David C. Christiani5,6
1Pathology, Massachusetts General Hospital, Charlestown, MA, United States, 2Charite Medical University, Berlin, Germany, 3Julius-Maximilians University, Wuerzburg, Germany, 4Pathology, Massachusetts General Hospital, Boston, MA, United States, 5Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, United States, 6Department of Medicine, Massachusetts General Hospital, Boston, MA, United States
1Pathology, Massachusetts General Hospital, Charlestown, MA, United States, 2Charite Medical University, Berlin, Germany, 3Julius-Maximilians University, Wuerzburg, Germany, 4Pathology, Massachusetts General Hospital, Boston, MA, United States, 5Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, United States, 6Department of Medicine, Massachusetts General Hospital, Boston, MA, United States
Synopsis
To improve the survival rate of non-small cell lung cancer (LuCa) patients, an economically efficient screening method without radiation hazard is preferred. Based on our previously reported potential serum biomarkers for predicting survival and differentiating LuCa stages and types, we investigated the potential for these biomarkers to be used for early detection screening using LuCa serum samples from patients prior to their diagnoses and compared with those measured at the time of diagnosis. If developed, our findings could be used as a minimally-invasive early diagnosis scheme.
INTRODUCTION
Lung cancer (LuCa) is currently the leading cause of cancer deaths in humans (1). Early stages are mostly asymptomatic (2) and contribute to a delayed diagnosis, with over 70% of the patients dying from lung cancer (1). While the overall five-year survival rate is 19% (1), LuCa detected at stage I can achieve a much higher five-year survival rate of 56% (1), which emphasizes the essential need for an affordable screening test. The latest method for the best possible early detection of LuCa is a low-dose spiral CT, but due to its uneconomical costs and radiation hazard it is inadequate as a widespread screening method (3). A simple blood sampling on the other hand provides good features for a screening method with being minimally-invasive, cost effective and without radiation exposure. Earlier we detected tissue and serum metabolic markers that can be used for survival estimation in early stage LuCa as well as for differentiation in typing and staging (4). Illustrated in Figure 1, our results show that metabolomic profiles can better differentiate LuCa from controls and between different cancer type than any single metabolites. These successes encouraged us to compare individual patient serum samples prior to LuCa diagnosis with their own samples at the time of diagnosis to examine potential serum metabolomic biomarkers for early detection.METHODS
Samples. Since 85% of all LuCa cases are histologically non-small cell lung cancer (NSCLC) (5), we focused on this histological type. We acquired serum samples from the Massachusetts General Hospital BioBank. Patients with serum samples from prior to and the date of a primary NSCLC diagnosis were selected and matched with healthy controls of the same age, gender and smoking habit. MR Spectroscopy. High resolution magic angle spinning (HRMAS) MRS analysis of tissue and serum samples are performed at 4°C by a 600MHz Bruker spectrometer at 3,600Hz spinning rate. Spectra are analyzed with a MatLab-based curve fitting program.RESULTS
Our population (n=81) consists of 25 NSCLC patients with serum samples from 0.5 to 5 years prior to diagnosis and additional serum samples of the date of diagnosis from the same patients. Each patient is matched to a healthy control according to age, gender and smoking habit. Furthermore, additional 56 patients with serum samples from 0 to 2 years prior to NSCLC diagnosis were identified as a testing cohort for validations. Figure 2 shows the distribution in age, gender and UICC8 LuCa stage of the population. Within NSCLC there are four histopathological subtypes (adenocarcinoma (AC) n=60, squamous cell carcinoma (SCC) n=15, adenosquamous cell carcinoma (ASC) n=2, mixed-carcinoma (MC) n=2) (Figure 3). Two NSCLC diagnosis could not be assigned to one subtype and are listed as n/a. The average number of days between the blood sampling and the diagnosis are also displayed in Figure 3. Figure 4 demonstrates the spectra of a serum sample of a 68 yo female patient with SCC at the time of diagnosis, compared with her serum sample 1150 days prior to diagnosis, and with a control subject. For instance, major differences in lipid and lactate levels and minor changes in PCh/GPG are clearly seen. Detailed analyses for the entire population are currently underway in our laboratory.DISCUSSION AND CONCLUSION
Encouraged by our observations of serum metabolomic markers in differentiating clinical and pathological differences for symptomatic patients, our current studies will advance the benefits of these markers as screening tools for the detection of early stage LuCa, to meet the need of this urgent clinical challenge. The current studies utilize the rich clinical resources provided by our institute that collects human serum samples prior to the diagnosis of diseases. If successful, as a serum screening protocol it can identify patients with suspicious results to undergo further radiologic examinations to detect LuCa at early stages.Acknowledgements
NIH grants CA141139 and the A.A. Martinos Center for Biomedical Imaging.References
- Siegel, R. L., Miller, K. D. and Jemal, A. (2019), Cancer statistics, 2019. CA A Cancer J Clin, 69: 7-34. doi:10.3322/caac.21551
- Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: Biology and treatment options. Biochim Biophys Acta. 2015;1856(2):189–210. doi:10.1016/j.bbcan.2015.08.002
- Moyer VA, US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160: 330-338–8
- Berker Y, Vandergrift L.A., Cheng LL. MRS based Metabolomic Biomarkers for Typing, Staging,and Survival Estimation of Early-Stage Human Lung Cancer. Scientific Reports 9, 10319 (2019), doi: 10.1038/s41598-019-46643-5
- Kilgoz H.O, Bender G., Scandura J.M., Viale A, Taneri B. KRAS and the Reality of Personalized Medicine in Non-Small Cell Lung Cancer, Molecular Medicine 22, 380-387 (2016), https://doi.org/10.2119/molmed.2016.00151
Figures
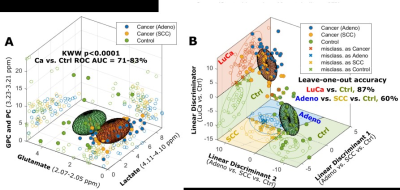
FIGURE 1: 3D ellipsoids generated from the
three panels in (A) that cover the volume of 3D Mahalanobis distance ≤1 (one
standard deviation from the centroid along each axis) from the class means for
LuCa and control groups, according to the covariance matrix of all class-mean-corrected
samples. (B) Two-class (LuCa vs. control) and three-class (Adeno vs. SCC vs.
control) linear discriminations calculated from 19 spectral regions (4).
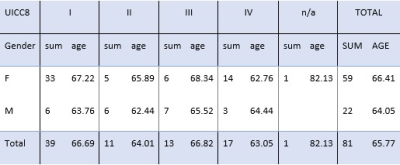
FIGURE 2: Population (n=81) with UICC8 stage (I=39, II=11, III=13,
IV=17, n/a=1), Gender (M=22, F=59) and age. Age is defined as average age on
the day of the NSCLC diagnosis (sd=8.68 years).
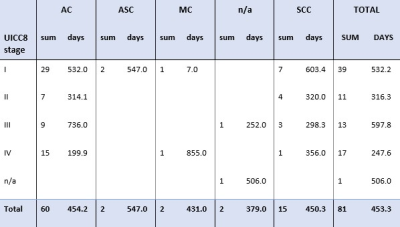
FIGURE 3: Pathological
data with UICC 8 stage of samples (n=81). AC=
Adenocarcinoma (n=60), ASC= Adenosquamous cell carcinoma (n=2), MC= mixed-cell
carcinoma (n+2), n/a= non-assigned subtype (n=2), SCC= squamous cell
carcinoma (n=15). SUM= count of population. Days= average number of days
between sample and diagnosis (sd=489.6 days).
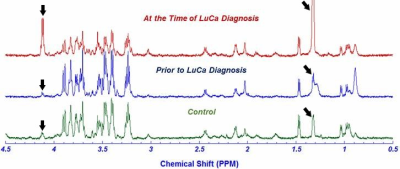
FIGURE 4: NMR Spectra 68 yo female, former smoker with SCC detected 1150 days after blood sample, matched with healthy
control of same age, gender and smoking habit. Major difference can be seen
in lactate level, corresponding to the peaks at 4.11 - 4.1ppm and 1.34 -
1.33ppm (ARROW) where lactate is significantly elevated prior to the diagnosis.