2950
GSH and GABA decreases in IDH1 mutated low-grade gliomas detected by HERMES spectral editing at 3 T in vivo1Shandong Medical Imaging Research Institute, Jinan, China, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
IDH1 mutation could result in better prognosis compared with wild-type, but the mechanisms remain largely unknown. GABA and GSH are low-concentration metabolites, could not be detected using conventional MRS, but playing an important role in energy production of glioma. HERMES can be thought of as two MEGA-PRESS experiments with the benefit of saving half the acquisition time to simultaneously detect GABA and GSH. The results demonstrated that HERMES is a reliable tool for the simultaneous detection of GABA and GSH signals, and both of them decreased significantly in IDH1 mutated low-grade gliomas.
Introduction
IDH1 mutational status has been proved to be an important prognostic biomarker in gliomas, and IDH1 mutation could result in better prognosis in low-grade gliomas compared with wild-type, but the mechanisms remain largely unknown1,2. vivo γ-aminobutyric acid (GABA) and glutathione (GSH) are low-concentration metabolites in brain, could not be detected using conventional MRS, but have an important role in energy production through substrate oxidation and defending oxidative stress, which is related with the neoplastic behavior of a tumor. Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy (HERMES) applies orthogonal editing encoding , can be thought of as two different MEGA-PRESS experiments being acquired at the same time, GSH- and GABA-edited difference spectra can be reconstructed from a single multiplexed experiment 3,4. So the purpose of this study was to examine in vivo GABA and GSH alterations in isocitrate dehydrogenase 1 (IDH1) mutated low-grade gliomas using HERMES.Methods
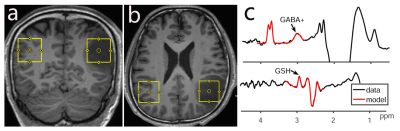
Fourteen patients with suspected diagnosis of low-grade glioma were enrolled prospectively in this study; all subjects underwent a 3T MRI scan, including 3D T1 weighted imaging and HERMES acquisition with a volume of interest 3×3×3 cm3. The parameters of HERMES were as follows: TR/TE 2000/80 ms, 320 averages, ~10 min per acquisition. The Regions of interest (ROIs) for HERMES were set at tumor foci and contralateral cerebral regions as controls (Fig.1). The GABA signal detected by HERMES also contains signal from macromolecules and homocarnosine, so it is referred to as GABA+. The detected GABA+ and GSH signals in tumor foci and contralateral cerebral regions were quantified using Gannet. The fitting errors and SNR of HERMES for GABA + and GSH were analyzed, FWHM of Water was also recorded. Paired t-test was performed to compare the GABA+ and GSH levels and FitError/SNR values between the tumor foci group and contralateral regions group in IDH1-mutant low-grade gliomas.Results
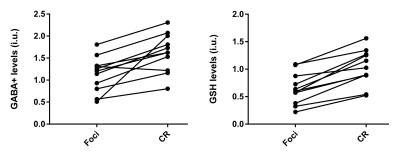
Histopathologic finding indicated that one case was inflammatory pseudotumor (IPT), not a tumor. The genotyping assay for IDH1 result showed 13 IDH1-mutant and 1 IDH1 wild-type cases, and another IDH1-mutant one is classified to high-glioma (WHO III) according to the histopathological result. Eleven IDH1-mutant low-grade gliomas were finally enrolled this study. HERMES could perform simultaneous measurement of GABA+ and GSH levels in glioma patients, FWHM of water was 9.67 ± 2.28%, all smaller than 15%, and no differences in FitError/SNR values (indicative of data quality) were found between glioma foci and contralateral regions (p=0.71 /0.51 and p=0.74/0.52 for GABA+ and GSH respectively). GABA+ and GSH both decreased in low-grade glioma foci – significant differences were found between tumor foci and contralateral regions of IDH1-mutant low-grade glioma patients (p=0.015, t=3.322; p=0.000, t=7.313 respectively), as shown in Fig.2.Discussion
HERMES of GABA and GSH 3, first proposed in 2016, can be thought of as two MEGA-PRESS experiments with the benefit of saving half the acquisition time to simultaneously detect GABA and GSH levels. The key point of HERMES is that editing pulses can be separately applied to GABA spins at 1.9 ppm and GSH at 4.56ppm. Most studies 5-8 applying HERMES have involved further methodological development, and the method has not yet been widely applied for clinical studies. So, this is the first study which evaluated the capability of HERMES in detecting the GABA and GSH alterations in gliomas.The results demonstrated that the GABA and GSH levels all decreased significantly in the region of tumor foci compared with contralateral cerebral regions. GABA is the main inhibitory neurotransmitter in the human brain and is present in high concentrations in presynaptic terminals of neuronal cells, serving a key role in shaping and regulating patterns of neuronal activity. Similarly, Lower GABA levels in tumors than normal brain has been reported in animal studies 9,10. One hypothesis 11 about GABA decrease is the absence of mature and/or well-differentiated neurons and glial cells in these tumors; and the IDH-mutated status would further decreased the GABA levels 12.
GSH is the most abundant redox compound in brain, serving an important role in minimizing the damage caused by reactive oxygen species. Deficits of GSH were seen in many neuropsychiatric and neurodegenerative disorders, and often occur earlier than other pathological abnormalities of the disease. One human study by Sotirios observed significantly reduced GSH levels in IDH mutant gliomas using MR spectra at 9.4T 13, which was consistent with the results of this study. However, one study found no differences in GSH levels between GBM and normal brain [9], and even GSH increased in another study 12. To our knowledge, this is the first time conducted to determine GSH variations in low-grade gliomas using 3T MRI.
Conclusion
HERMES can noninvasively detect GABA and GSH alterations in patients with low-grade glioma with IDH1 mutation; it may be a valuable tool in the search for latent biomarkers for future characterization of low-grade glioma. Noninvasive detection of GSH and GABA may prove to be a valuable diagnostic and prognostic biomarker of IDH1-mutant low-grade glioma.Acknowledgements
We would like to thank all patients for their participation.References
1. Dubbink HJ, Taal W, van Marion R, et al. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73(21):1792-1795.
2. Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(25):4150-4154.
3. Saleh MG, Oeltzschner G, Chan KL, et al. Simultaneous edited MRS of GABA and glutathione. NeuroImage. 2016;142:576-582.
4. Chan KL, Oeltzschner G, Saleh MG, Edden RAE, Barker PB. Simultaneous editing of GABA and GSH with Hadamard-encoded MR spectroscopic imaging. Magnetic resonance in medicine. 2019.
5. Berrington A, Barker PB, Edden RAE, Mikkelsen M. Frequency and phase correction for multiplexed edited MRS of GABA and glutathione. Magnetic resonance in medicine. 2018;80(1):21-28.
6. Gong T, Harris AD, Oeltzschner G, et al. Hadamard editing of glutathione and macromolecule-suppressed GABA. Magnetic resonance in medicine. 2018;31(1).
7. Saleh MG, Mikkelsen M. Simultaneous editing of GABA and glutathione at 7T using semi-LASER localization. 2018;80(2):474-479.
8. Saleh MG, Rimbault D, Mikkelsen M, et al. Multi-vendor standardized sequence for edited magnetic resonance spectroscopy. NeuroImage. 2019;189:425-431.
9. Hulsey KM, Mashimo T, Banerjee A, et al. (1)H MRS characterization of neurochemical profiles in orthotopic mouse models of human brain tumors. NMR in biomedicine. 2015;28(1):108-115.
10. Lai M, Vassallo I, Lanz B, et al. In vivo characterization of brain metabolism by (1) H MRS, (13) C MRS and (18) FDG PET reveals significant glucose oxidation of invasively growing glioma cells. International journal of cancer. 2018;143(1):127-138.
11. Faria AV, Macedo FC, Jr., Marsaioli AJ, Ferreira MM, Cendes F. Classification of brain tumor extracts by high resolution (1)H MRS using partial least squares discriminant analysis. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2011;44(2):149-164.
12. Jalbert LE, Elkhaled A, Phillips JJ, et al. Metabolic Profiling of IDH Mutation and Malignant Progression in Infiltrating Glioma. Scientific reports. 2017;7:44792.
13. Bisdas S, Chadzynski GL, Braun C, et al. MR spectroscopy for in vivo assessment of the oncometabolite 2-hydroxyglutarate and its effects on cellular metabolism in human brain gliomas at 9.4T. Journal of magnetic resonance imaging : JMRI. 2016;44(4):823-833.
Figures