Sónia Isabel Gonçalves1, Rui V. Simões1, and Noam Shemesh1
1Champalimaud Research, Champalimaud Centre for the Unknown, Lisbon, Portugal
Synopsis
MRS allows non-invasive in-vivo exploration of tissue metabolism.
However, about half of the proton spectrum (downfield of water) has been nearly
ignored over the decades of MRS application due to water suppression. We show
that ISIS-based Relaxation Enhanced MRS (iRE-MRS) which uses frequency
selective excitation and ISIS localization offers short echo times and enhances
exchange-broadened resonances. For the first time, we measure in-vivo downfield
spectra in mouse glioma tumors (and controls) and show remarkable spectral signatures
for the tumor downfield.
Introduction
Downfield magnetic resonance spectroscopy (MRS) has received little
attention insofar due to the difficulty in detecting resonances mostly
originating from labile protons. Only a few studies with high dynamic range MRS
[1-5] or Relaxation-Enhanced MRS [6] (RE-MRS), focused on downfield spectra [7,
8], and revealed (i) interesting resonances undetectable in the upfield
counterpart (NAD NADH) [9] and (ii) exchange-related phenomena [5]. A recently
introduced method, termed iRE-MRS [1], combines RE-MRS [6] with
Image-Selected-In-Vivo-Spectroscopy (ISIS) localization [10] in the aim of
obtaining downfield spectra at TEs as short as 5 ms. Given the rapidly
exchanging signals downfield, we hypothesized that short TE downfield spectra
may provide insight in disease in general and in tumors in particular, where
changes in environment may lead to differences in metabolic concentrations but
also perhaps exchange properties. Here, we use iRE-MRS to investigate, for the
first time, in-vivo downfield MRS spectra in glioma tumors in-vivo.Methods
Animal experiments were preapproved by the
institutional and national authorities and carried out according to European
Directive 2010/63. Experiments were carried out on wild type mice, N=4 control
(4 males, mean weight = 21.2±1.3 g) and N=5 tumors (2 males, mean weight =
20.9±1.3 g), aged ~5-6 months.
Animal
preparation. Tumors were induced in
all N=5 animals by intracranial stereotactic injection of 10e5 GL261 cells in the
caudate nucleus as reported previously in [11].
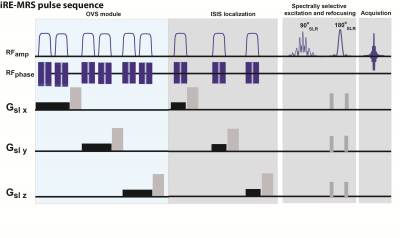
Pulse sequence design.
Figure 1 illustrates the iRE-MRS pulse sequence. It is based on
spectrally-selective excitation and refocusing, avoiding water suppression [5].
However, unlike the previous RE-MRS studies, localization is achieved by an
ISIS block, consisting of three spatially-selective adiabatic inversions. OVS
modules were implemented to improve localization quality.
Spectrally-selective RF pulses. The
spectrally-selective RF pulses were generated by the shape algorithm
implemented in Paravision 6.0.1 (Bruker Biospin, Ettlingen, Germany), which
employs the Shinar LeRoux algorithm [12]. Excitation and refocusing pulses were
centered at 9.5 ppm, having a bandwidth of 6.5 ppm.
MRS experiments. All
experiments took place approximately 2.5 weeks after injection and were
performed using a 9.4 T horizontal bore scanner (Bruker-Biospin, Karlsruhe,
Germany), equipped with an 86 mm coil for transmission and a 4-element array
cryocoil (Bruker BioSpin, Fallanden, Switzerland) for signal reception. iRE-MRS
spectra were obtained in a 2.2×2.2×2.2 mm3 voxel, located in the
striatum. Experiments were executed using the following common acquisition
parameters: TR=15000 ms (>>5T1 of metabolites), 8 steps per ISIS cycle,
15 repetitions (cycles) per experiment, spectral width 5597.01 Hz, spectral
resolution 1.94Hz/point.
Post-processing. Each FID (ISIS
cycle) was Fourier transformed, the resulting spectra were individually
rephased and then averaged over the total number of repetitions for each scan. Apodization
was carried-out with an exponential factor of 20 Hz-width. All spectra were
scaled to the amplitude of the unsuppressed water peak and aligned with respect
to the ‘NAA’ amide peak falling at approximately 7.8 ppm [13].Results
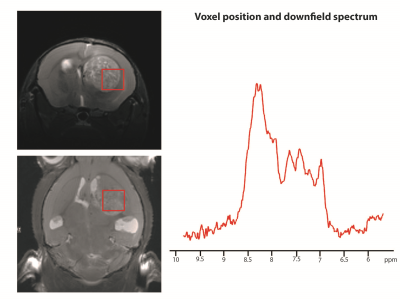
Figure 2 shows the voxel positioning and downfield
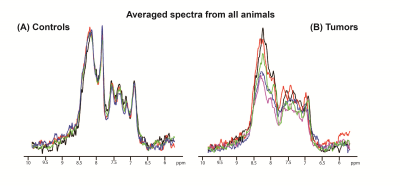
spectrum in one illustrative animal. Figure 3 shows individual
overlaid raw iRE-MRS spectra for controls (Figure 3a) and tumors (Figure 3b).
Visible differences between controls and tumors can be observed in all spectral
peaks, especially with respect to peak amplitude and to some extent, also
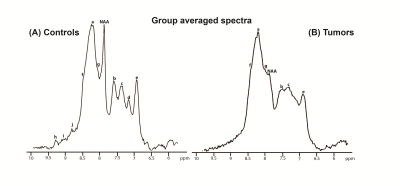
alignment. Figure 4 shows the average spectra spectrum over animals for both
controls (Figure 4a) and tumors (Figure 4b). The average spectrum for controls
closely reproduces previous results in the rat [14], with peaks labeled as “NAA”, a, b, c, d
and e easily distinguishable. Other
smaller peaks, located at ~8.5 (f),
8.25 (g), and 9.0 (j), 9.3 (i), and 9.5 (h) ppm are
also apparent. For tumors, the average spectrum shows evident differences:
strong attenuation of peaks “NAA”, b, c,
and e, while peaks j, i,
and h completely disappear.Discussion
Our results indicate that in-vivo downfield spectra of glioma tumors are
sensitive to the disease through marked spectral changes. Tumor growth involves
a complex interplay of factors [15-17] that comprise, amongst others, disturbed
metabolism, and acidification of the cellular environment which can influence
the exchange rates and peak locations of exchanging protons [8]. Our findings
already suggest quite a few interesting targets for future research: (1) Peaks j, i
and h, which probably correspond to
the NAD+ proton resonances [9], completely disappear in tumor, suggesting redox
potential changes in the tumor area [18]; (2) We observed peak location shifts
which may represent pH effects [8]. Such hypothesis can be tested in the future
by employing water-suppression and observing how exchange impacts these signals
directly [6]. Finally, though more research is required in terms of spectral
assignment, the changes observed here may differ in different types of tumors.
All these vistas augur well for downfield MRS as a sensitive marker for cancer.Conclusion
In-vivo downfield iRE-MRS of glioma tumors revealed marked spectral
differences indicating features other than those typically associated with
upfield counterparts, such as changes in redox potential and pH. These findings
strongly motivate future studies exploring downfield MRS signatures.Acknowledgements
The work of Rui V. Simões was funded by grant H2020-MSCA-IF-2018, ref 844776.References
[1] Gonçalves et al., ISMRM 2018; [2] Piotto et al., J. Biomol. NMR, 2, 661-665, 1992; [3] Dreher and
Leibfritz, Magn. Res. Med., 54, 190-195, 2005. [4] MacMillan et al., 65,
1239-1246, 2011; [5] MacMillan et al., 70, 916-924, 2013; [6] Shemesh
et al., Nat Commun, doi:
10.1038/ncomms5958, 2014; [7] van Zijl and Moonen,
Magn. Res. Med., 29: 381-385, 1993; [8] Mori et al., Magn. Res. Med., 40,
36-42, 1998; [9] Graaf and Behar Magn. Res. Med., 27, 802-809, 2014; [10] Ordidge
et al., J. Magn. Res., 66, 283, 1986; [11] Simões et al., NMR
Biomed, 21, 251-264, 2008; [12] Pauly et
al., IEEE Trans. Med. Imaging, 10, 53-65, 1991;
[13] Fichtner at al., Magn. Res. Med., 78, 11-19, 2017 [14] Gonçalves et al.,
Magn. Res. Med., 82(4), 1266-77, 2019; [15] Korenjak and Zavadil, Cancer Sci.,
Oct 8, 2019; [16] Hsieh and Tsai, World J Gastrointest Oncol, 11(9): 686-704,
2019; [17] Markopoulos et al., Cells, 8(19), 2019; [18] Acharya et al., Oxid
Med Cell Longev, 3(1), 23-34, 2010.