2939
Association of multiple sclerosis central fatigue with inhibitory and excitatory neurotransmitters1School of Health Sciences, University of Newcastle, Newcastle, Australia, 2Imaging centre, Hunter Medical Research Institute, Newcastle, Australia, 3The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 5HMRI Imaging centre, Hunter Medical Research Institute, Newcastle, Australia, 6School of Biomedical Sciences, Queensland University of Technology, Brisbane, Australia, 7Faculty of Health and Medicine, University of Newcastle, Newcastle, Australia, 8Department of Neurology, John Hunter Hospital, Newcastle, Australia
Synopsis
Despite neuro metabolic and morphological alterations linked to central fatigue in multiple sclerosis (MS), the pathophysiology of this symptom is not fully understood. Dysfunction of the GABAergic/Glutamatergic pathways involving inhibitory and excitatory neurotransmitters such as γ-aminobutyric acid (GABA) and glutamine+glutamate pools (Glx) have been implicated in several neurological disorders, including MS. In this study, we evaluated if GABA and Glx levels are associated with central fatigue in MS. Our results showed significant correlations of GABA and Glx levels with fatigue scores which suggest dysregulation of GABAergic/glutamatergic neurotransmission is possibly implicated in the mechanisms of mediating central fatigue in MS
Introduction
Fatigue is a common symptom in patients with multiple sclerosis (MS)1. Although previous studies suggest metabolic and morphological alterations in the brain, the pathophysiology of MS fatigue remains unclear2-4. γ-aminobutyric acid (GABA) and glutamine+glutamate (Glx) are the primary inhibitory/excitatory neurotransmitters responsible for regulating many physiological processes5,6. Dysfunction of the GABAergic/glutamatergic pathways has been implicated in several neurological disorders such as depression, pain, schizophrenia and MS5-12. In particular, altered levels of GABA in the sensory motor cortex have been correlated with physical disability in relapse onset MS13,14. Association between MS-related fatigue and elevated levels of glutamate has also been reported suggesting dysfunction in the glutamatergic pathway15. Although changes in GABA and glutamate metabolisms may play important roles in the control of cortical excitability, their role in fatigue development in MS is less known. The purpose of this study was therefore to evaluate the potential role of these neurometabolites in a group of stable randomly selected relapse remitting MS (RRMS) patients with central fatigue.Methods
The local ethics review board approved this study and all subjects were consented in writing prior to undertaking any study assessments. Sixteen RRMS patients (mean age 34.1±7.7 years), and thirteen age and gender-matched healthy controls (HC) were scanned on a 3T MR system, equipped with 64 channel brain coil (Magnetom Prisma, Siemens Healthineers). None of RRMS group was receiving any medications that could potentially affect GABA levels and their expanded disability status scale (EDSS) ranged from 0-4. Fatigue levels were assessed using Modified Impact Fatigue Scale (MFIS) that comprised cognitive, physical and psychosocial domains of fatigue. The GABA and Glx levels were collected from right pre-frontal cortex (PFC) and sensorimotor cortex (SMC) using MEscher-GArwood (MEGA-PRESS) editing sequence with TR/TE: 2000/68ms, voxel size: 18.75 (PFC)/15.62mls (SMC). The editing pulses were centered at 1.9ppm to collect signal from CH2 moiety close to creatine at 3ppm. An unsuppressed water reference scan was also acquired with the same parameters immediately after the editing sequence. The spectral analysis was undertaken using Gannet 3.1.16. In addition to GABA+ (GABA, macromolecules and homocarnosine) and Glx concentration levels, N-acetylaspartate (NAA) and creatine (Cr) were also quantified from off-resonance spectra using LCmodel17. Metabolite ratios to Cr were then calculated. Group mean difference (Mann Whitney U test) and correlation (Spearman rho) statistical analyses were carried out using SPSS.Results and Discussion
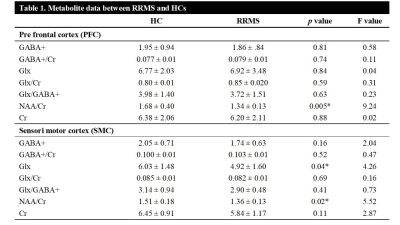
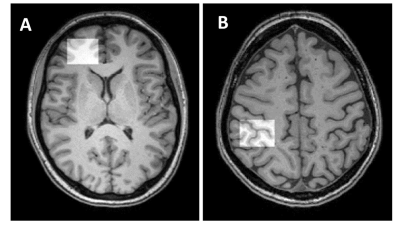
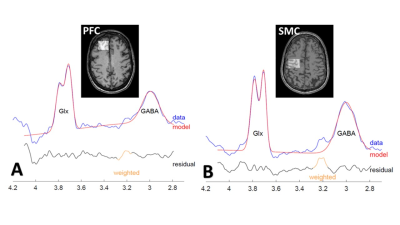
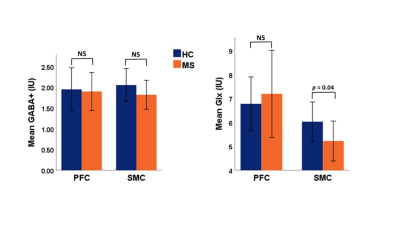
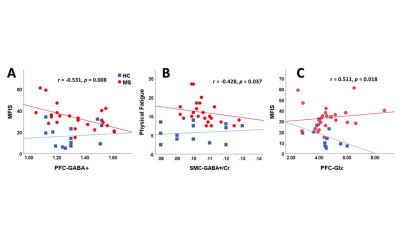
The mean values of clinical fatigue scores and metabolite ratios are shown in Table 1. Figure 1 shows MRS voxels from PFC and SMC from a RRMS patient. Figure 2 shows modelling of GABA and Glx signals with Gannet fit module. Compared with HCs, RRMS had significantly higher fatigue scores and statistically significant reduction in Glx in SMC (p <0.04) (Figure 3). The RRMS showed significantly lower NAA/Cr ratio relative to healthy control (p <0.02) in both voxels. CSF corrected and creatine scaled GABA+ levels were lower in both locations in MS compared to HC cohort, however, difference did not reach statistical significance. RRMS group showed significant reduction of CSF corrected Glx in SMC (p = 0.04) but not in PFC, compared to HC. MFIS showed moderate but negative correlations with GABA+ levels in PFC (r = -0.472-0.531, p ≤ 0.020) and positively with PFC Glx (r = 0.480-0.511, p ≤ 0.028). However, GABA+/Cr ratio in SMC showed negative correlation with physical fatigue (r = -0.428, p = 0.037) (Figure 4). The marked decrease in GABA+ levels in examined voxels could represent dysregulated GABAergic pathway in MS patients as reported in other studies14,18. Increased levels of Glx in PFC may reflect elevated metabolic turnover from increased activity of cytokines that are known to block reuptake, and release of glutamate by astrocytes via several pathways including in chronic fatigue syndrome19,20.Conclusion
GABA+ and Glx may play a role in the pathogenesis of fatigue. Our results suggest dysregulation of GABAergic/glutamatergic neurotransmission is possibly implicated in the mechanisms of mediating central fatigue in MS.Acknowledgements
The authors acknowledge the patients and healthy controls who volunteered to take part in this study and the Imaging Centre of the University of Newcastle and Hunter Medical Research Institute.
Funding for this study was through an independent investigator-initiated grant provided by Hunter Medical Research Institute.
References
1.Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs 2003;17(4):225-234.
2. Zaini WH, Giuliani F, Beaulieu C, Kalra S, Hanstock C. Fatigue in Multiple Sclerosis: Assessing Pontine Involvement Using Proton MR Spectroscopic Imaging. PLoS One 2016;11(2):e0149622.
3.Tellez N, Alonso J, Rio J, et al. The basal ganglia: a substrate for fatigue in multiple sclerosis. Neuroradiology 2008;50(1):17-23.
4.Pokryszko-Dragan A, Bladowska J, Zimny A, et al. Magnetic resonance spectroscopy findings as related to fatigue and cognitive performance in multiple sclerosis patients with mild disability. J Neurol Sci 2014;339(1-2):35-40
5.De Stefano N, Giorgio A. GABA: a new imaging biomarker of neurodegeneration in multiple sclerosis? Brain 2015;138(Pt 9):2467-2468.
6.Nantes JC, Proulx S, Zhong J, et al. GABA and glutamate levels correlate with MTR and clinical disability: Insights from multiple sclerosis. Neuroimage 2017
7. Petroff OA. GABA and glutamate in the human brain. Neuroscientist 2002;8(6):562-573.
8.Yalcinkaya N, Akcan U, Orcen A, et al. Reduced fecal GABA levels in multiple sclerosis patients. Mult Scler Relat Disord 2016;9:60-61.
9.Cleve M, Gussew A, Reichenbach JR. In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. Neuroimage 2015;105:67-75.
10.Fayed N, Andrés E, Viguera L, Modrego PJ, Garcia-Campayo J. Higher Glutamate + Glutamine and Reduction of N-acetylaspartate in Posterior Cingulate According to Age Range in Patients with Cognitive Impairment and/or Pain. Academic Radiology 2014;21(9):1211-1217.
11.Kim GH, Kang I, Jeong H, et al. Low Prefrontal GABA Levels Are Associated With Poor Cognitive Functions in Professional Boxers. Frontiers in Human Neuroscience 2019;13(193).
12. Menschikov PE, Semenova NA, Ublinskiy MV, et al. (1)H-MRS and MEGA-PRESS pulse sequence in the study of balance of inhibitory and excitatory neurotransmitters in the human brain of ultra-high risk oschizophreniaf patients. Dokl Biochem Biophys 2016;468(1):168-172.
13.Cawley N, Solanky BS, Muhlert N, et al. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 2015;138(9):2584-2595.
14.Bhattacharyya PK, Phillips MD, Stone LA, Bermel RA, Lowe MJ. Sensorimotor cortex gamma-aminobutyric acid concentration correlates with impaired performance in patients with MS. Am J Neuroradiol 2013;34(9):1733-1739.
15.Kantorova E, Polacek H, Bittsansky M, et al. Hypothalamic damage in multiple sclerosis correlates with disease activity, disability, depression, and fatigue. Neurol Res 2017;39(4):323-330.
16.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging 2014;40(6):1445-1452.
17.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001;14(4):260-264.
18.Bhat R, Axtell R, Mitra A, et al. Inhibitory role for GABA in autoimmune inflammation. Proceedings of the National Academy of Sciences 2010;107(6):2580-2585.
19.Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res 2007;85(10):2059-2070.
20.
Y. Nakatomi, K. Mizuno, A. Ishii, Y. Wada, M. Tanaka, S. Tazawa, K.
Onoe, S. Fukuda, J. Kawabe, K. Takahashi, Y. Kataoka, S. Shiomi, K. Yamaguti,
M. Inaba, H. Kuratsune, Y. Watanabe, Neuroinflammation in Patients with Chronic
Fatigue Syndrome/Myalgic Encephalomyelitis: An (1)(1)C-(R)-PK11195 PET Study, J
Nucl Med 55(6) (2014) 945-50.
Figures