2932
Proton magnetic resonance spectroscopy (1H-MRS) in degenerative cervical spinal cord compression.1Departement of Medical Imaging and Image-guided Therapy, High Field MR Center, Vienna, Austria, 2Masaryk University, Brno, Czech Republic, 3Department of Neurology, University Hospital Brno, Brno, Czech Republic, 4Department of Internal Medicine, Medical University of Vienna, Vienna, Austria, 5Multimodal and Functional Imaging Laboratory, Central European Institute of Technology, Brno, Czech Republic, 6Departments of Neurology and Biomedical Engineering, University Hospital Olomouc, Olomouc, Czech Republic, 7Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
While cervical spinal cord (CSC) compression occurs almost ubiquitously with aging, early metabolic changes in CSC that might develop into irreversible clinical myelopathy symptoms have not been described yet. Thus, we utilized fine-tuned semi-LASER 3T MRS protocol that addresses limitations of standard MRS methods to unravel metabolic damage in the cranial parts of the CSC in 50 patients with non-myelopathic degenerative cervical cord compression and 10 with clinically symptomatic degenerative cervical myelopathy in comparison to 35 healthy controls. Higher tNAA/tCr and myo-Ins/tNAA levels suggest axonal loss above the level of stenosis, indicating that CSC impairment exceeds the level of compression.
Introduction
While cervical spinal cord (CSC) compression occurs almost ubiquitously with aging,1 early structural and metabolic changes in CSC that might develop into irreversible clinical myelopathy symptoms have not been described yet. Although current MRS methods provide a comprehensive description of neurochemical profiles,2,3 to date, the number of MRS studies in CSC compression remained limited due to technical challenges that compromise spectral quality. Thus, we utilized fine-tuned semi-LASER 3T MRS protocol that addresses limitations of standard MRS methods to unravel metabolic damage in the cranial parts of the CSC in patients with non-myelopathic (asymptomatic) degenerative cervical cord compression (NMDCCC) and clinically symptomatic degenerative cervical myelopathy (DCM) in comparison to healthy controls (HC).Methods
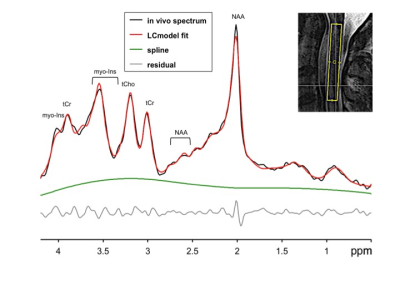
MRS data were acquired at 3T Siemens-Prisma with a standard 64 channel head-neck coil utilizing single-voxel cardiac-triggered semi-LASER4 localization sequence (256 NEX, TR=5s, TE=28ms, AT=15 min.) with implemented prospective scan-to-scan frequency correction, water, and outer volume suppression, and FOCI refocusing pulses with excellent localization performance. MRS 3.2 mL voxel was placed above the level of stenosis, i.e., C1-C3 in 50 NMDCCC (55.3±10.1 y.o., 28 males), 10 DCM (58.6±4.7 y.o., 5 males) and 35 HC (52.3±10.3 y.o., 15 males). Magnetic field inhomogeneities were minimized using FASTMAP. Spectra were processed in MRSpa software and quantified utilizing LCmodel. Neurochemical profiles referenced to unsuppressed water spectrum, and selected metabolite ratios were compared among groups with ANOVA and post-hoc tests with Tukey correction. Patients were screened for abnormalities in motor and sensory evoked potentials (EP).Results
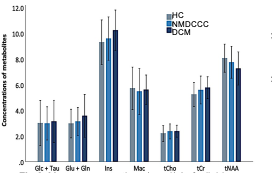
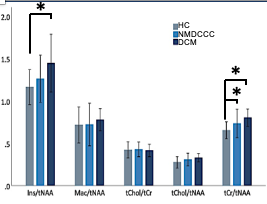
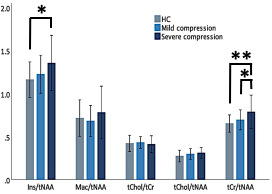
Mean SNR of metabolite spectra of 13.2±2.9 (provided by LCmodel) and linewidth of the unsuppressed-water spectrum 12.9±3.0 Hz illustrate high quality of MRS data (Fig. 1). While individual metabolites with mean CRLB<20% only displayed non-significant trends among groups (Fig. 2), total creatine (tCr)/total N-acetylaspartate (tNAA) distinguished HC from NMDCCC and DCM, myo-inostitol (myo-Ins)/tNAA ratio was significantly higher in patients with DCM than in HC (Fig .3). In addition, myo-Ins/tNAA and tCr/tNAA distinguished HC from patients with severe CSC compression (compression ratio<0.4 and cross-sectional area<71 mm2, N=35) and tCr/tNAA was significantly higher in patients with severe than in mild compression (N=32) (ANOVA + Tukey, Fig.4). No significant differences in metabolite profiles were found between patients with (N=25) and without (N=26) EP abnormity (standard two-tailed unpaired t-test). No significant relationship between metabolite levels and age (Pearson’s correlation) was observed.Discussion
Higher tNAA/tCr and myo-Ins/tNAA levels suggest axonal loss due to Wallerian-like degeneration above the level of stenosis, indicating that SC impairment exceeds the level of compression. This corroborates other studies showing neurochemical deficits in the primary sensorimotor cortex in the brain,5,6 as well as in patients with CSC injury and more severe clinical impairment.2 Significant changes in NMDSCC suggest microstructural impairment even at early stages of degenerative compression which is consistent with DTI findings.7 Abnormalities of sensory and motor EP in NMDCCC as predictors of DCM development likely reflects different aspect of microstructural impairment than neurochemical profiling.Conclusions
Optimized MRS at standard 3T scanner provides high spectral quality and thus offers biochemical markers, which relate to the severity of compression and clinical manifestation. Their clinical relevance needs to be established in longitudinal studies assessing the risk of myelopathy development in NMDCCC, highly prevalent in population.1Acknowledgements
Ministry of Health of the Czech Republic, NV18-04-00159. NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (Grant No. 27238) and European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 846793 to PB and No. 794986 to AS.References
1.Kovalova.Spine 2016;41:1908.
2.Wyss.Radiology 2019;291:131.
3.Lenglet. Proc of the Hum Brain Mapp 2016; 1245.
4.Deelchand.Magn Reson Med 2015;73:1718.
5.Aleksanderek.Radiology 2017;282:817.
6. Gohmann. Eur J Radiol 2019;116:55.
7. Valosek. ISMRM 2019; 304.
Figures