2929
1H MRS at 3T reveals reduced GABA and glutamate levels in the visual cortex induced by pharmacologically increased dopamine1Center for Stroke Research Berlin, Charité – Universitätsmedizin Berlin, Berlin, Germany, 2Department of Psychiatry and Psychotherapy, Charité – Universitätsmedizin Berlin, Berlin, Germany
Synopsis
Schizophrenia frequently manifests psychotic symptoms, such as delusions and hallucinations. However, its neurochemical mechanisms are not well deciphered, though dysfunctional dopaminergic neurotransmission is suggested. In this study, single volume 1H MRS using MEGA-PRESS at 3 T was applied to investigate possible neurochemical changes in the visual cortex induced by pharmacologically increased dopamine levels in healthy volunteers that also performed a visual detection task. Increased dopamine yielded decreased GABA and reduced glutamate quantities. Furthermore, an inverse correlation of glutamate with false perceptions in the detection task supports the theory that glutamate hypofunction might contribute to the formation of hallucinations in schizophrenia.
Purpose
Recently, the effect of increased dopamine levels on the inhibitory neurotransmitter γ-aminobutric acid (GABA) in the visual cortex using 1H MRS in the context of schizophrenia was investigated1. Schizophrenia is characterized by psychotic symptoms, such as delusions and hallucinations. Despite of intense research efforts, its neurochemical underpinnings are not sufficiently understood. Converging evidence suggests a link between psychotic symptoms and a dysfunction in dopaminergic neurotransmission. For instance, antipsychotic drugs function as dopamine receptor antagonists, and PET studies demonstrate increased dopamine synthesis in the striatum2. On the other hand, several findings point to aberrations in different transmitter systems. Based on the observation that antagonists of the glutamate NMDA receptor induce schizophrenia-like symptoms, a disruption of glutamatergic pathways has been implicated in the etiology of schizophrenia3. Similarly, altered GABA levels have already been linked to psychotic conditions and to related perceptual perturbations4,5. Thus, it is important to elucidate interactions between these transmitter systems and their relationships to psychotic core symptoms, such as hallucinations. The aim of this study was to use L-DOPA to directly modulate the dopamine system of healthy human individuals while they engage in a task that was designed to measure hallucination-like misperception of illusory faces in noise and to investigate the effect of increased dopamine levels on neurotransmitters in the visual cortex using 1H MRS. New results and correlations of metabolite levels with task performance are reported.Methods
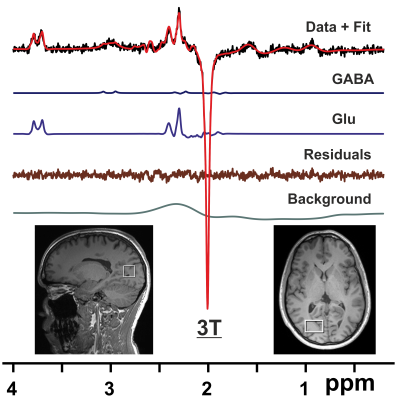
Fourteen subjects (aged 26 – 48 yrs, 9 f) were scanned twice with a minimum interscan interval of one week (one subject was excluded from analysis due to poor data quality, hence N = 13). One hour prior to each scanning session, subjects were administered orally either 150 mg of L-DOPA or a placebo. All parties involved were blinded with respect to the randomly assigned substance administration. Scans were performed on a 3T Trio system (Siemens Healthineers, Erlangen, Germany) using a 32 channel receive only RF coil (N = 9), and after an upgrade on a 3T PrismaFit system employing a 64 channel receive only RF coil (N = 4). NMR data were acquired as described previously1 including high-resolution MPRAGE imaging for voxel placement, localized RF calibration, vendor-supplied shimming, and the use of an automatic alignment algorithm to ensure the same voxel positioning in both sessions. Single volume spectra from the right visual cortex were measured using the MEGA-PRESS technique6 (VOI = 20x30x20 mm3, TR/TE = 3000/68 ms, number of averages = 128, Tacq = 1024 ms, and editing pulse at 1.9 ppm). Pre-processing of MR spectra was performed using the FID-A toolkit7. Metabolites of the resulting difference spectra were quantified using LCModel8 with a simulated basis set. Results from MRS were compared for scan sessions “Placebo” and “L-DOPA” using a paired t-test. In addition, subjects were asked to detect faces in a total of 360 noisy pictures, half of which contained actual faces. Details of this task are described in a preprint9, where it was also shown that individuals with a high proneness for hallucinations in questionnaires asking for anomalous perceptions, tend to detect more face in images without actual face (false positives). To test if transmitter alterations, which have been implicated in the formation of psychosis, are related to such hallucination-like perception, metabolite levels for GABA and glutamate (Glu) for the two sessions were correlated with the false positive rate from the described face detection task.Results
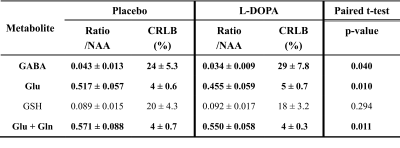
Shimming resulted in water linewidths of 7.2 ± 0.5 Hz across all scans. Metabolite quantification of MEGA-PRESS difference spectra (Fig. 1) yielded significant reductions (p<0.05) of the mean ratios of GABA, Glu, and combined Glu + glutamine (Gln) with respect to N-acetylaspartate (NAA) for the session “L-DOPA” compared to session “Placebo” (Table 1). In addition, absolute Glu levels were inversely correlated with the false positive rate in the face detection task (Fig. 2), suggesting that individuals with low glutamate levels in the visual cortex showed more hallucination-like false positives.Discussion
In this study, the dopamine system of healthy volunteers was altered by the administration of L-DOPA compared to placebo. Significant differences in metabolite ratios of the visual cortex, in particular for neurotransmitters GABA and Glu, were observed using 1H MRS. Dopamine-related reduction in GABA levels might thus partially explain perceptual deficits seen in schizophrenia (as hypothesized previously, e.g. with respect to decreased contrast sensitivity10). Moreover, the finding of an increased number of hallucination-like false positives in individuals with smaller Glu quantities is in line with the theory of glutamate hypofunction in schizophrenia that was based on the observation that both, antiglutamatergic drugs and antibodies, instantly induce psychosis-like symptoms3. The result that L-DOPA administration reduced glutamate levels, which were, in turn linked to increased hallucinatory perception, might contribute to bridging the gap between glutamate and dopamine theories of hallucinations.Conclusion
Reduced GABA and Glu levels in the visual cortex as assessed by an 1H MRS editing scheme were found when dopamine levels were increased with an oral administration of L-DOPA. In addition, an inverse correlation of Glu levels with hallucination-like false perceptions in a visual detection task supports the theory that glutamate hypofunction might contribute to the formation of hallucinations in schizophrenia.Acknowledgements
No acknowledgement found.References
1. Mekle R, Schmack K, Fiebach JB, Stuke H. Pharmacologically increased dopamine levels reduce GABA and glutamate concentrations in visual brain areas – a 1 H MRS study at 3T. ISMRM 27th Annual Meeting. Montreal, QC, Canada: International Society of Magnetic Resonance in Medicine, 2019: 2257.
2. Howes OD, Nour MM. Dopamine and the aberrant salience hypothesis of schizophrenia. World psychiatry : official journal of the World Psychiatric Association 2016;15:3-4.
3. Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2012;37:4-15.
4. Marsman A, Mandl RC, Klomp DW, et al. GABA and glutamate in schizophrenia: a 7 T (1)H-MRS study. NeuroImage Clinical 2014;6:398-407.
5. Yoon JH, Maddock RJ, Rokem A, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. The Journal of neuroscience : the official journal of the Society for Neuroscience 2010;30:3777-3781.
6. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine 1998;11:266-272.
7. Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med 2017;77:23-33.
8. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672-679.
9. Stuke H, Kress E, Weilnhammer VA, Sterzer P, Schmack K. Overly strong priors for socially meaningful visual signals in psychosis proneness. bioRxiv 2018:473421.
10. Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Archives of general psychiatry 1999;56:149-154.
Figures