2924
Association of brain metabolite concentrations and pain perception in a cohort of patients with chronic pain
Maame Owusu-Ansah1, Candace C. Fleischer1, and Daniel E. Harper2
1Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 2Department of Anesthesiology, Emory University School of Medicine, Atlanta, GA, United States
1Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 2Department of Anesthesiology, Emory University School of Medicine, Atlanta, GA, United States
Synopsis
A proof-of-concept generalized linear model to characterize the relationships between brain metabolite concentrations and pain perception is presented, explicitly accounting for differences in regional grey matter (GM) density. Brain metabolite concentrations measured with magnetic resonance spectroscopy (MRS), and pain perception characterized using quantitative sensory testing (QST), were significantly associated in a cohort of patients with chronic pain. These results contribute to the expanding literature that supports the utility of neuroimaging to understand the underlying mechanisms of centralized chronic pain.
Introduction
Previous research to identify neuroimaging signatures of chronic pain has led to varied results. While morphological brain changes may contribute to the persistence and evolution of chronic pain, numerous reports have observed decreases while others observe increases in GM density as a function of chronic pain [1,2]. Similarly, the use of MRS to characterize neurochemical abnormalities, such as the dysregulation of neurotransmitters including glutamate, is an increasingly common approach. However, both increases and decreases in brain metabolites have been reported, resulting in a limited understanding of the role of brain metabolites in pain perception. Taken together, there is a clear need for improved non-invasive MR biomarkers for prognostication and treatment monitoring to further explore the underlying mechanisms that drive chronic centralized pain. Here, we present a proof-of-concept approach using a generalized linear model to characterize changes in brain metabolites in a cohort of temporomandibular disorder (TMD) patients with chronic pain.Methods
A schematic of the study design is shown in Figure 1. After providing written informed consent, MR data and quantitative sensory testing (QST) metrics were acquired in TMD patients (n=16) and healthy controls (HCs; n=12). MR data was acquired on a 3T MR scanner (GE Signa) using a 32-channel receive array head coil. T1-weighted SPGR (TR/TE = 650/3.7 ms, flip angle = 8°, voxel resolution = 0.5x0.5x0.4 mm3, NEX = 1) and single-voxel PRESS MRS (TR/TE = 1500/35 ms, flip angle = 90°, complex data points = 4096, 2 cm isotropic voxel in the anterior cingulate cortex, ACC) were acquired. MR spectra were analyzed with LCModel (v6.3-1L) [3]. Tissue metabolite concentrations were corrected for cerebrospinal fluid (CSF) after segmentation with FSL-FAST [4]. FSL-VBM (http://www.fmri-b.ox.ac.uk/fsl/) [5-7] was used to quantify GM morphometry in the ACC region using the following steps: 1) Brain tissue was extracted using BET [8]. 2) A study-specific template was formed using a randomly chosen subset of subjects (11 HCs and 11 TMD patients) and nonlinear registration using FNIRT, image averaging, tissue segmentation, and affine registration to the MNI152 template using FLIRT [9]. 3) Individual GM images were nonlinearly registered to the template and modulated with a Jacobian of the warp field. 4) These images were then smoothed with a Gaussian kernel with sigma of 7 mm. 6) Finally, an ACC region of interest (ROI) mask was generated and used to calculate regional GM density (mm3) for each subject using the individual images that were registered to the study-specific template. Quantitative sensory testing (QST) metrics were used to quantify pain perception and included: current face pain as a marker for chronic pain symptoms; symptom severity using the widespread pain index and fibromyalgia-ness scores; fibromyalgia determined with the American College of Rheumatology’s 2011 Fibromyalgia Criteria; pressure algometry in the trapezius and knee muscles; and cuff algometry using a blood pressure cuff inflated to 160 mm Hg. Statistical analysis was performed in SPSS v26 (IBM) using generalized linear models. Significance was determined at p<0.05.Results and Discussion
Descriptive statistics are shown in Table 1. Significant differences in metrics of centralized pain including current face pain, fibromyalgia score, and symptom severity were observed in TMD patients compared to HCs. Age and sex were not significantly different between groups. Initial analysis included linear regressions of glutamate+glutamine (Glx) with QST metrics in TMD and HCs (Tables 2,3). Significant associations of Glx with QST metrics were observed when accounting for GM density in TMD patients (GLM regressions, Table 2). Inclusion of GM density in the ACC ROI as an additional linear covariate in our model resulted in improved model fits for TMD patients. In comparison, Glx was not significantly associated with most QST metrics in healthy controls (Table 3). Brain metabolite concentrations are known to vary as a function of GM volume within and between subjects [10]. As a result, GM volume or density must be considered as a potential covariate when characterizing changes in metabolites, particularly as GM volumes have been observed to significantly vary with symptom severity. Significant model associations between Glx and pain sensitivity in the knee and trapezius muscles using algometry were observed, suggesting that dysregulation of brain metabolites may be associated with heightened pain perception. While some studies that account for differences in GM use average GM concentrations as a correction factor, or MRS voxel segmentation to quantify GM fractions, the use of morphometry is an alternative correction approach presented here. The benefit of incorporating GM density from morphometry, rather than GM fractions from voxel segmentation, is that morphometry accounts for differences in brain size and local concentration variations between subjects and does not assume GM metabolite concentrations a priori. Importantly, previous discrepancies in reported metabolite concentrations may be attributed to variations in GM morphometry. Explicitly including GM density as a covariate in the model can account for tissue differences at the subject level.Conclusion
Improved predictions of the association between brain metabolites and pain perception was facilitated with a generalized linear model that accounts for differences in GM density. Morphometry may be a promising alternative to voxel segmentation to perform tissue composition corrections when quantifying brain metabolite changes in chronic pain.Acknowledgements
This work was supported in part by NIH K99DE026810.References
- Smallwood, RF et al. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain. 2013;14(7):663-75.
- Cauda, F et al. Grey matter alterations in chronic pain: A
network-oriented meta-analytic approach. Neuroimage Clin. 2014;4:676-686.
- Provencher, SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672-379
- Zhang Y, Brady, M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57.
- Douaud, G et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375-2386.
- Good, CD et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21-36.
- Smith, SM et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208-219.
- Smith, SM. Fast robust automated brain extraction. Hum Brain Mapp, 2002;17(3):143-55.
- Jenkinson, M and Smith, S, A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156.
- Tal, A et al. The role of grey and white matter segmentation in quantitative proton MR spectroscopic imaging. NMR Biomed. 2013;25(12):1392-1400.
Figures
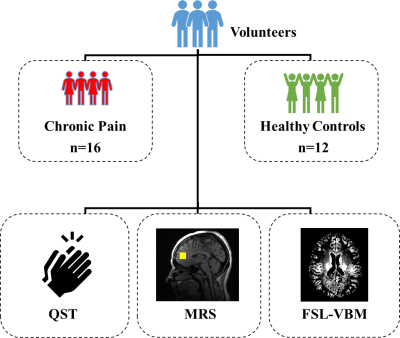
Figure
1. Schematic overview of study design. Temporomandibular
disorder patients with chronic pain and healthy controls underwent quantitative
sensory testing (QST), MRI, and MRS.
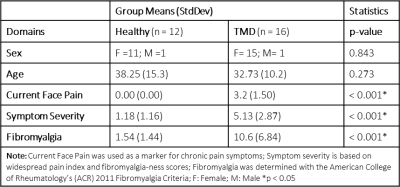
Table 1. Descriptive statistics of
temporomandibular disorder patients and healthy controls included in the study.
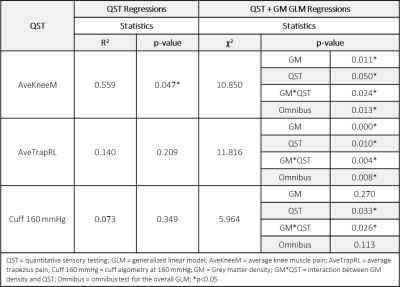
Table 2: Associations between glutamate + glutamine
(Glx) and QST metrics in TMD patients. QST regressions consider only the effect
of the QST on Glx concentrations. Incorporating the additional covariate of
grey matter density using a generalized linear model results in improved model
predictions.
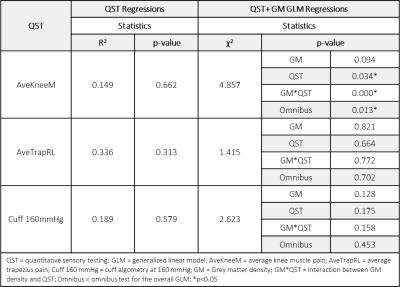
Table 3: Associations between glutamate + glutamine
(Glx) and QST metrics in healthy controls. QST regressions consider only the
effect of the QST on Glx concentrations. Incorporating the additional covariate
of grey matter density using a generalized linear model results in improved
model predictions. Compared to TMD patients in Table 2, only a single QST was
significantly associated with Glx concentrations after accounting for grey
matter density.