2923
Detection of acetyl-carnitine in the human heart in vivo using long echo-time 1H-MRS at 3T1Oxford Centre for Clinical Magnetic Resonance Research, RDM Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom, 2Department of Physics, University of Oxford, Oxford, United Kingdom, 3Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford, United Kingdom, 4Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom, 5Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom, 6Department of Imaging Methods, Slovak Academy of Sciences, Bratislava, Slovakia
Synopsis
Cardiac acetyl-carnitine plays an important role in fat metabolism. Decreased carnitine concentration has been reported in heart failure, but it has previously been difficult to measure acetyl-carnitine in-vivo. We show for the first time that it is possible to detect a clear acetyl-carnitine resonance at δ=2.1ppm without lipid contamination within the heart using a long echo time semi-LASER sequence at 3T. This validates the finding in skeletal muscle that the T2 of acetyl-carnitine is much longer than its surrounding lipid signals. Further work is underway to investigate variability of the acetyl-carnitine signal postprandially.
Introduction:
Carnitine and acetyl-carnitine play an important role in fat metabolism [1]. Carnitines act as a required shuttle of acetyl groups into the mitochondrial matrix for beta-oxidation, and, through the inhibition of carnitine acyltransferase by malonyl-CoA, is implicated in the pathogenesis of insulin resistance [2].While healthy heart is able to oxidise, in order of preference, fats, sugars and ketone bodies to power contraction, the failing heart loses this flexibility and predominantly utilises glycolysis. Decreased carnitine concentrations have been reported in heart failure [3], and supplementation shown to have positive effects on cardiac function in rat hearts [4], but the ability to measure cardiac acetyl-carnitine in vivois extremely limited.1H-MRS using long echo-times allows measurements of acetyl-carnitine in skeletal muscle [5, 6]. Therefore, our aim was to test the feasibility of cardiac acetyl-carnitine detection using long echo-time 1H-MRS at 3T.Methods:
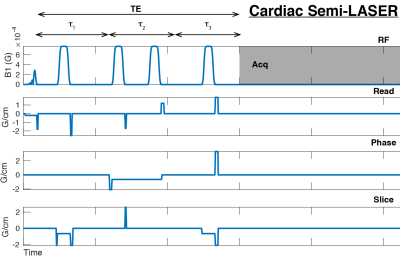
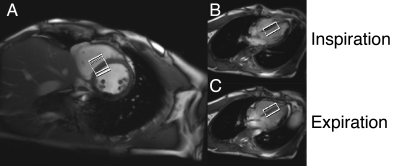
Four healthy volunteers (3M/1F, 31±5 years, 22.6±5.2 kg.m-2) were recruited for this study and after informed consent were scanned supine in a 3T Siemens (Erlangen, Germany) Trio MR scanner equipped with a 32ch body array (Siemens).A previously described semi-LASER localised spectroscopy sequence [7] was used for 1H-MRS data acquisition (Figure 1) . The voxel of interest was placed such that as much as possible of the interventricular septum was contained within the voxel regardless of either the cardiac or respiratory cycles (Figure 2), exploiting echo formation for blood-pool and motion suppression. Each volunteer was hence instructed to breathe normally throughout the 1H-MRS acquisition.Two sets of cardiac 1H-MRS spectra were acquired using the semi-LASER sequence, one with a relatively short total echo time ($$$\tau_1$$$=$$$\tau_2$$$=$$$\tau_3$$$=25 ms; total TE 75 ms) and second using a long echo time ($$$\tau_1$$$=$$$\tau_2$$$=$$$\tau_3$$$=50 ms; total TE 150 ms). Other scan parameters were as follows: 3 s TR, 1.5 kHz BW, 192 averages, acquisition time 9 minutes. No water suppression scheme was applied.The short TE scan with higher SNR were used to find optimum coil combination weights for WSVD combination [8], which were then applied to process the data from the long TE scans. After coil combination, individual signal transients that exhibited larger than 1.3 standard deviation difference to the mean spectrum were discarded and the remaining spectra were frequency aligned, using the residual water peak.Results:
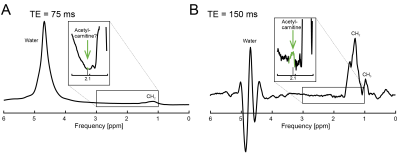
Acetyl-carnitine signal resonating at 2.1 ppm was clearly differentiable from surrounding signals in all participants. Figure 3 depicts representative short echo-time and long echo-time spectra. A clear acetyl-carnitine signal, without lipid contamination, is visible in the long echo-time spectrum.Discussion:
In our study we have applied an approach of using long echo-time single voxel 1H-MRS for in vivo acetyl-carnitine measurement, which was previously validated in skeletal muscle [5], in the human heart. This allowed us for the first time to detect acetyl-carnitine in the healthy human heart in vivo. We were at this point not able to perform absolute quantification as the long echo-time acquisition requires correction for T2 signal decay, which is currently unknown for cardiac acetyl-carnitine, and will be a target of future work. However, it is clear from our results that the T2 of acetyl-carnitine is much longer than T2 of its surrounding lipid signals, which is in good agreement with previous skeletal muscle experiments [5, 6].While our relatively large voxel size (30 mL) and ungated acquisition could potentially lead to higher blood contamination, a separate control acquisition with a shorter number of averages indicated that water signal was not changed by cardiac gating. This hence allows for a free breathing acquisition, preferred as the required number of averages would be unfeasible to acquire even in multiple breath holds. A navigated sequence could potentially be used in the future [9]. Any variability of cardiac acetyl-carnitine levels during the day, as demonstrated in skeletal muscle [6], needs to be investigated.Conclusion:
We have successfully demonstrated the feasibility to detect cardiac acetyl-carnitine in vivo using long echo-time 1H-MRS at 3T. This work paves way to investigations of acetyl-carnitine levels in failing heart and potential validations of carnitine supplementation for improvement in cardiac function in humans.Acknowledgements
JP would like to thank the Rokos Foundation for an undergraduate Research Studentship.
JJM would like to thank the support of a Novo Nordisk Postdoctoral Fellowship, a Junior Research Fellowship at Wadham College, Oxford, and a Stipendiary Lectureship in Physics at St Hugh's College, Oxford. CTR and LV are funded by a Sir Henry Dale Fellowship from the Wellcome Trust [098436/Z/12/B]. All authors would like to thank the British Heart Foundation for funding (refs. FS/14/17/30634, FS/16/7/31843). The support of the Slovak Grant Agencies VEGA [2/0001/17] and APVV [#15–0029] is also gratefully acknowledged. JJM and LV are both last authors of this work.
References
1. Karlic, H. and A. Lohninger, Supplementation of L-carnitine in athletes: does it make sense?Nutrition, 2004. 20(7-8): p. 709-15.
2. Mingrone, G., Carnitine in type 2 diabetes.Ann N Y Acad Sci, 2004. 1033: p. 99-107.
3. Liedtke, A.J., S.H. Nellis, and L.F. Whitesell, Effects of carnitine isomers on fatty acid metabolism in ischemic swine hearts.Circ Res, 1981. 48(6 Pt 1): p. 859-66.
4. Savic, D., et al. L-Carnitine Shows Beneficial Effects on Cardiac Metabolism and Function: A Hyperpolarized MRS and Langendorff Perfusion Study. in Proc. Intl. Soc. Mag. Reson. Med. 26. 2018. Paris, France.
5. Lindeboom, L., et al., Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection.J Clin Invest, 2014. 124(11): p. 4915-25.
6. Klepochova, R., et al., Detection and Alterations of Acetylcarnitine in Human Skeletal Muscles by 1H MRS at 7 T.Invest Radiol, 2017. 52(7): p. 412-418.
7. Berrington, A., et al., Improved localisation for 2-hydroxyglutarate detection at 3T using long-TE semi-LASER.Tomography, 2016. 2(2): p. 94-105.
8. Rodgers, C.T. and M.D. Robson, Receive array magnetic resonance spectroscopy: Whitened singular value decomposition (WSVD) gives optimal Bayesian solution.Magn Reson Med, 2010. 63(4): p. 881-91.
9. Schar, M., S. Kozerke, and P. Boesiger, Navigator gating and volume tracking for double-triggered cardiac proton spectroscopy at 3 Tesla.Magn Reson Med, 2004. 51(6): p. 1091-5.
Figures