2919
Ultrafast diagonal-suppressed 2D MR correlation spectroscopy1Philips Healthcare, Beijing, China, 2Department of Electronic Science, Xiamen University, Xiamen, China
Synopsis
Two-dimensional (2D) MR spectroscopy enables feasible detection of low-concentration scalar-coupled metabolites in human tissues. However, the traditional acquisition of 2D MRS can be time consuming, and cross peaks which contain crucial spectral information in 2D correlated spectra (COSY) usually get contaminated by nearby strong diagonal peaks. Here, a pulse sequence is developed for ultrafast recording of diagonal-free 2D MR correlated spectra, thus allowing improved recognition and measurement of scalar-coupled metabolites.
Introduction
Advantages of two- (2D) over one-dimensional (1D) MR spectroscopy lie in the improved spectral resolution and the enriched spectral information that benefit measurement of low-concentration scalar(J)-coupled molecules in biological tissues. In order to construct the indirect dimension with a decent resolution, numerous transients with progressive evolution delays should be collected in the traditional acquisition of 2D MRS, resulting in expensive time cost. Various methods have been previously proposed for fast 2D MRS. Among them, the SECOT method 1 spatially encoded the indirect-dimensional spectral information with a spatially dependent spin selective excitation (the so-called ‘Zangger-Sterk (ZS)’ excitation 2), and then the echo planar spectroscopic imaging (EPSI) acquisition module 3 is used after the mixing period for repeatedly decoding of the encoded spectral information, thus allowing acquisition of 2D spectral information within a single shot. Besides, cross peaks in 2D 1H homonuclear correlated spectra (COSY), that contain crucial spectral information, are usually contaminated by nearby strong diagonal peaks. Here, a pulse sequence (UF-DS-COSY) based on the ‘ZS’ excitation is developed for ultrafast recording of diagonal-suppressed 2D correlated spectra and thus improved detection of scalar-coupled metabolites. The proposed method is in principle applicable to different kinds of 2D homonuclear correlation experiments.Methods
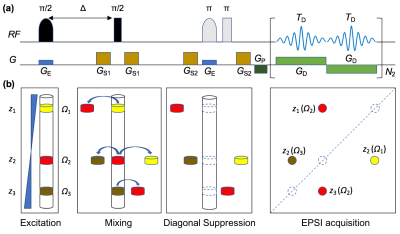
The proposed UF-DS-COSY pulse sequence is shown in Fig. 1(a), and a schematic diagram is provided in Fig. 1(b) for understanding of the signal evolution. We consider a hypothetical molecule containing three protons with different chemical shifts, among which proton 2 (Ω2) is coupled to both protons 1 (Ω1) and 3 (Ω3) whereas proton 1 is not directly coupled with proton 3. In the excitation period, protons 1-3 are respectively excited in different slices (z1-z3). The following constant-time Δ introduces a certain accumulation of J coupling evolutions. After the mixing period, magnetizations of individual protons are partially transferred to their coupling partners. The untransferred magnetizations (corresponding to the unwanted diagonal peaks) will then be spoiled by the following diagonal suppression (DS) module with only cross peaks (transferred magnetization) retained for final observation. The EPSI acquisition module is introduced at last for acquisition of 2D spectral information within a single-shot.The ZS module in the sequence will inevitably introduce sensitivity losses because only a fraction (Δωex/Δω) of signals from the detection region are excited and then evolve to be detected, where Δωex is the excitation bandwidth of the selective π/2 pulse and Δω the frequency dispersion introduced by the encoding gradient GE. Besides, spectral widths in both dimensions (SW1 = Δω = γGEL and SW2 = 1/(2TD)) will be contradictory with the digital resolution in F1 dimension (Δν1 = GE/(GDTD)), where γ is gyromagnetic ratio of proton, L the detection length along the encoding direction, and GD and TD the strength and duration of each decoding gradient. Therefore, trade off should be made among the spectral widths and resolutions when carry out the UF-DS-COSY experiment. Additionally, molecule diffusion along the spatial encoding direction during signal evolution and data acquisition may result in residual diagonal resonances and broadened spectral width along the indirect dimension in the final 2D COSY.
Experiments on a mixed metabolite solution were performed on an 11.7 T Agilent NMR System (Agilent Technologies, Santa Clara, CA, USA). Metabolites including N-acetyl aspartate (NAA), γ-aminobutyric acid (GABA), creatine (Cr), myo-inositol (mI), and taurine (Tau) were dissolved in water (95% D2O) with an identical concentration of 0.1 M. Experimental parameters used for the previous SECOT-COSY and/or the proposed UF-DS-COSY were set as: durations of the selective π/2 and π pulses were 50 and 22 ms respectively, GE = 0.3 G/cm, Δ = 30 ms, GS1 = 20 G/cm, GS2 = 15 G/cm, duration of GS1 and GS2 1.5 ms, GD = 30 G/cm, TD = 250 us, data points in both dimensions 60 and 300, number of average samplings = 32, and total acquisition time 2.7 min. All 2D correlated spectra were displayed in magnitude mode.
Results and Discussion
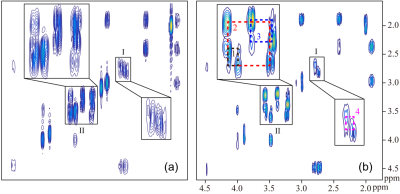
Results for the metabolite solution are shown in Fig. 2. We can see from the SECOT-COSY that cross and diagonal peaks are overlapped in the crowded spectral regions (See square I and II in Fig. 2(a)), thus hampering recognition of relevant cross peaks. With suppression of diagonal peaks, the cross peaks (including 1 and 2: mI, 3: Tau, and 4: NAA) can be much more reliably identified and measured in the UF-DS-COSY spectrum in Fig. 2(b). Residual diagonal signals in Fig. 2(b) should be resulted from diffusion of metabolite molecules along the encoding direction.The proposed pulse sequence suffers from sensitivity losses that are majorly resulted from the ‘ZS’ (slice-selective) excitation, thus requiring increased number of average samplings. Alternatively, monochromatic pulses in the 'ZS' modules can be replaced with polychromatic pulses 4 for improved sensitivity performance, but it places a requirement that resonances in the spectrum are well separated for multi-band excitation. In addition, the hyper-polarization techniques 5 may also benefit applications of the proposed method with enhanced signal intensities.
Conclusion
In summary, a pulse sequence is designed for ultrafast recordings of 2D diagonal-suppressed MR correlated spectra, allowing improved recognition and measurement of scalar-coupled metabolites.Acknowledgements
No acknowledgement found.References
1. Ye Q, Chen L, Qiu W, et al. Accelerating two-dimensional nuclear magnetic resonance correlation spectroscopy via selective coherence transfer. J Chem Phys. 2017;146:014202.
2. Zangger K, Sterk H. Homonuclear broadband-decoupled NMR spectra. J Magn Reson. 1997;124:486-489.
3. Posse S, DeCarli C, Le Bihan D. Three-dimensional echo-planar MR spectroscopic imaging at short echo times in the human brain. Radiology. 1994;192:733-738.
4. Zhang Z, Smith PE, Frydman L. Reducing acquisition times in multidimensional NMR with a time-optimized Fourier encoding algorithm. J Chem Phys. 2014;141:194201.
5. Frydman L, Blazina D. Ultrafast two-dimensional nuclear magnetic resonance spectroscopy of hyperpolarized solutions. Nat Phys. 2007;3:415-419.
Figures