2911
B0 Inhomogeneity Corrected Reconstruction for Low-Resolution J-resolved MRSI Using Low-Rank and Spatial Constraints1Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 2Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States
Synopsis
This work presents a new method for B0 inhomogeneitycorrected reconstruction of J-resolved (multi-TE) MRSI datawith limited k-space coverage. A constrained reconstruction formulation was proposed to simultaneously reconstruct the data at all TEs, using a joint low-rank constraint that exploits the spatial-spectral-TE correlation in the data and a spatial constraint that exploits prior edge information from high-resolution anatomical images. Simulation and in vivo experiments using a customized 3D J-resolved EPSI acquisition have been performed to evaluate the proposed method. Experimental results demonstrated improved lineshape and SNR achieved by the proposed method over existing B0 correction methods.
Introduction
J-resolved (or Multi-TE) MRSI encodes the J-coupling of different molecules and allows for improved spectral separation and quantification1-3. However, the need of acquiring data at multiple TEs leads to multiplied acquisition time, thus further limiting the trade-offs among SNR, speed and spatiospectral resolution. Although fast-scanning methods such as EPSI-based sequences can be used for acceleration, low-resolution data is typically acquired in practice to maintain a reasonable scanning time and SNR3-5. The increased voxel size makes the experiments more susceptible to B0 inhomogeneity, leading to undesirable lineshape distortions and SNR loss. The conjugate phase (CP) method4 can correct the B0-induced frequency shifts by performing reconstruction at higher-resolution grids than the acquired data to improve lineshapes. But its performance is often limited due to the inherent ill-posedness of the reconstruction problem. An overdiscrete reconstruction has been introduced to optimize the voxel spatial response functions thus minimizing the B0 effects6. Constrained reconstruction has been recently proposed as an alternative approach to address the ill-posed B0 correction problem7-9 for single-TE acquisitions while they can be applied to J-resolved MRSI by treating each TE independently, the performance degrades as TE increases (lower SNRs) and they do not effectively exploit the correlation in the multi-TE data. We proposed here a new method to reconstruct low-resolution J-resolved MRSI data with B0 correction, using joint multi-TE low-rank and spatial constraints. Simulation and in vivo studies have been conducted to evaluate the proposed method.Method
The data acquired in a J-resolved MRSI experiment can be expressed as:$$d(\mathbf{k},t_2,t_1)=\int \rho(\mathbf{r},t_2,t_1)e^{-i2\pi\Delta f(\mathbf{r})t_2}e^{-i2\pi\mathbf{kr}}d\mathbf{r}+n(\mathbf{k},t_2,t_1), \quad\quad\quad(1)$$ where $$$\rho(\mathbf{r},t_2,t_1)$$$ denotes the desired multi-TE spatiotemporal function, with $$$t_2$$$ the chemical shift encoding time (FID) and $$$t_1$$$ the J-coupling encoding time (TE), $$$d(\mathbf{k},t_2,t_1)$$$ denotes the acquired $$$(k,t)$$$-space data, $$$\Delta f(\mathbf{r})$$$ B0 inhomogeneity maps and $$$n(\mathbf{k},t_2,t_1)$$$ the noise. After discretization, Eq. (1) can be written in the following matrix representation:$$\mathbf{d_{t_1}}=\Omega\{\mathbf{FB}\odot\boldsymbol{\rho}_{t_1}\} + \mathbf{n_{t_1}}, \quad\quad\quad(2)$$where $$$\boldsymbol{\rho}_{t_1}$$$ is a Castrati matrix of the spatiotemporal function at each TE9, $$$\mathbf{B_{nm}}=e^{i2\pi\mathbf{r_n}t_{2,m}}$$$ (B0-induced linear phase), $$$\mathbf{F}$$$ the Fourier transform matrix with $$$\Omega$$$ a $$$k$$$-space truncation operator and $$$\mathbf{n}$$$ is the noise vector. Because the model in Eq. (2) is only accurate when the B0 inhomogeneity is almost constant within each voxel, $$$\boldsymbol{\rho}_{t_1}$$$ needs to be modeled on higher-resolution grids than that afforded by $$$\mathbf{d}_{t_1}$$$ to ensure sufficiently small voxel sizes, making the problem ill-posed.We describe here a constrained reconstruction method to address this challenge for multi-TE MRSI. Specifically, we model $$$\boldsymbol{\rho}_{t_1}$$$ using a long Casorati matrix $$$\boldsymbol{\rho}=[\boldsymbol{\rho}_{_{TE_1}},\boldsymbol{\rho}_{_{TE_2}},...,\boldsymbol{\rho}_{_{TE_N}}]$$$, where $$$\boldsymbol{\rho}_{_{TE_i}}$$$ denotes the matrix of the $$$i$$$-th TE. Inspired by the subspace model of $$$\boldsymbol{\rho}_{\mathbf{r},{t_2}}$$$ described in (10-11) and the resulting low-rank property of $$$\boldsymbol{\rho}_{_{TE_i}}$$$ (for a single $$$t_1$$$), we model $$$\boldsymbol{\rho}$$$ using an explicit low-rank decomposition: $$\boldsymbol{\rho}=\mathbf{UV}=\mathbf{U}[\mathbf{V}_{_{TE_1}},\mathbf{V}_{_{TE_2}},...,\mathbf{V}_{_{TE_N}}],\quad\quad\quad(3)$$ which indicates each $$$\boldsymbol{\rho}_{_{TE_i}}$$$ can be modeled as $$$\boldsymbol{\rho}_{_{TE_i}}=\mathbf{UV}_{_{TE_i}}$$$. Furthermore, we exploit a high-resolution anatomical image readily available in an MRSI experiment to derive prior spatial edge information to constrain the spatial variations in multi-TE spatiotemporal function of interest. Integrating these two constriants, we formulate the reconstruction as: $$\{\hat{\mathbf{U}},\hat{\mathbf{V}}\}=\arg\min_{\mathbf{U},\mathbf{V}}\|\mathbf{d}-F_{\Omega}\{\mathbf{B}\odot\mathbf{UV}\}\|^2_2+\lambda_1\|\mathbf{WDUV}\|^2_F,\quad\quad\quad(4)$$ where $$$\mathbf{d}$$$ is a vector containing the low-resolution data at all TEs, $$$F_{\Omega}$$$ the Fourier encoding operator with $$$k$$$ space truncation $$$\Omega$$$, $$$\mathbf{B}$$$ models B0 inhomogeneous effect (a concatenation of B matrices for different TEs). $$$\odot$$$ a point-wise multiplication operator, $$$\mathbf{W}$$$ contains the edge weights derived from anatomical images, and $$$\mathbf{D}$$$ is a finite difference operator. $$$\lambda_1$$$ is the parameter for a mild weighted-$$$\ell_2$$$ regularization. We solve this optimization problem by alternatively minimizing it w.r.t $$$\mathbf{U}$$$ and $$$\mathbf{V}$$$. Initial guesses of $$$\mathbf{U}$$$ and $$$\mathbf{V}$$$ are obtained by performing an SVD to a minimum-norm reconstruction. The rank was selected empirically by observing the singular value decay.
Results and Discussion
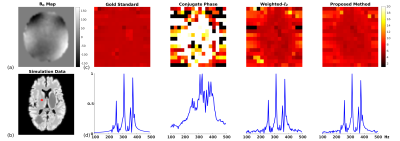
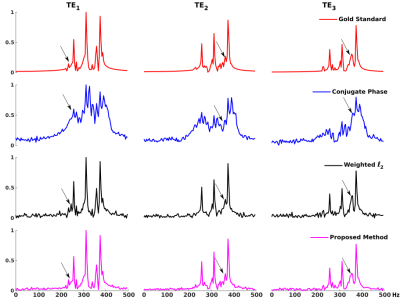
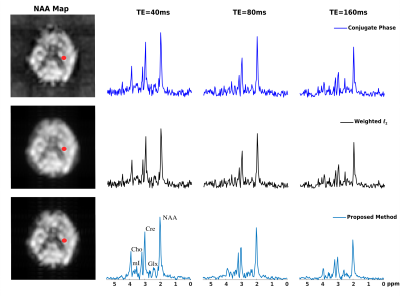
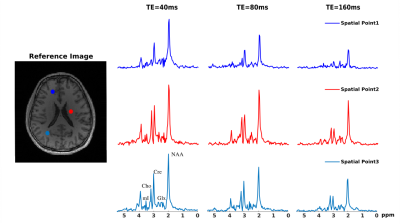
A computational J-resolved MRSI phantom (64×64voxels at 3 TEs) was simulated to validate the proposed method. The phantom contains spatially varying spectra of different molecules generated using GAVA12 and further combined with different ratios according to segmented regions(Fig. 1b). A high-resolution experimentally acquired B0 map was used to introduce the B0 effects (Fig. 1a). Noise was added at various peak SNR levels. Figures 1 and 2 compare the B0 corrected reconstruction from 16×16 k-space data by different methods, i.e., CP method, a weighted-$$$\ell_2$$$ method and the proposed method. As can be seen, the proposed method corrected B0-induced lineshape distortion better than alternative methods (Fig. 1) as well as offer extra SNR gains across all TEs (Fig. 2). In vivo data were acquired from healthy volunteers on a Siemens 3T scanner (Prisma) using a customized 3D J-resolved spin-echo EPSI sequence .The acquisition parameters are: FOV=220×220×64mm3, matrix size=24(kx)×20(ky)×8(kz), TR=1300ms, 3 TEs at 40, 80, 160ms, spectral BW=1200Hz and 256 gradient echoes for each TE. Results were compared for the CP reconstruction, weighted-$$$\ell_2$$$ regularized reconstruction and the proposed method (Figs. 3-4). Again, the proposed method produced B0-corrected reconstruction with the best spectral quality and SNR.Conclusion
We have developed a new method for B0 inhomogeneity corrected reconstruction from low-resolution J-resolved MRSI acquisitions. The proposed method jointly reconstruct the data at multiple TEs and solves this ill-posed problem using joint low-rank and spatial edge constraints. Both simulation and in vivo results demonstrated improved lineshape and SNR achieved by the proposed method over existing methods applied to individual TEs.Acknowledgements
No acknowledgement found.References
[1] de Graaf RA, In vivo NMR spectroscopy: Principles and techniques. Hoboken, NJ: John Wiley and Sons, 2007.
[2] Bolliger CS, Boesch C, and Kreis R, On the use of Cramér–Rao minimum variance bounds for the design of magnetic resonance spectroscopy experiments. NeuroImage, 2013;83:1031-1040.
[3] Li Y, Chen AP, Crane JC, Chang SM, Vigneron DB, and Nelson SJ, Three-dimensional J-resolved H-1 magnetic resonance spectroscopic imaging of volunteers and patients with brain tumors at 3T. Magn Reson Med, 2007;58:886-892.
[4] Adalsteinsson E, Spielman DM. Spatially resolved two-dimensional spectroscopy. Magn Reson Med. 1999;41(1):8-12
[5] Wilson NE, Iqbal Z, Burns BL, et al. Accelerated five-dimensional echo planar J-resolved spectroscopic imaging: Implementation and pilot validation in human brain. Magn Reson Med. 2016;75(1):42-51.
[6] Kirchner T, Fillmer A, and Henning A. Mechanisms of SNR and line shape improvement by B0 correction in overdiscrete MRSI reconstruction. Magn Reson Med 77.1 (2017): 44-56.
[7] Peng X, et al. "Correction of field inhomogeneity effects on limited k-space MRSI data using anatomical constraints." 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology. IEEE, 2010.
[8] Liu Y, Ma C, Clifford B et al., Improved Low-Rank Filtering of Magnetic Resonance Spectroscopic Imaging Data Corrupted by Noise and B0 Field Inhomogeneity. IEEE Trans Biomed Eng;63(4):841-849.
[9] Klauser, A, Courvoisier, S, Kasten, J, et al. Fast high‐resolution brain metabolite mapping on a clinical 3T MRI by accelerated 1H‐FID‐MRSI and low‐rank constrained reconstruction. Magn Reson Med., 2019;81:2841-2857.
[10] Liang ZP. Spatiotemporal imaging with partially separable functions. In IEEE International Symposium on Biomedical Imaging, Arlington, VA, USA, 2007. pp. 988–991.
[11] Lam F and Liang ZP, A subspace approach to high-resolution spectroscopic imaging. Magn. Reson. Med., 2014;71:1349-1357.
[12] Soher BJ, Young K, Bernstein A, Aygula Z, and Maudsley AA, GAVA: spectral simulation for in vivo MRS applications. J. Magn. Reson., 2007;185:291-299.
Figures