2906
Fast spectroscopic imaging of GABA at 7T
Jullie W Pan1, Victor Yushmanov1, Chan H Moon1, and Hoby P Hetherington1
1Radiology, University of Pittsburgh, Pittsburgh, PA, United States
1Radiology, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
We have developed and implemented a fast spectroscopic imaging acquisition for selective homonuclear polarization transfer sequence for the detection of GABA at 7T. With the high SNR available at 7T, whole slice studies of 1.65cc nominal resolution are achievable with acquisition durations (5 to 10min) conventionally used in single voxel studies. As this editing is performed with longitudinal magnetization, this method is single shot and is flexible for evaluation of multiple coupled spins.
Introduction
There are a number of strategies for detection of brain GABA, with the most common approach targeting the 3.0ppm C2 resonance through J-difference editing (1,2). We have previously described a longitudinal approach to GABA detection (3) premised on selective polarization transfer. This method provides single shot detection and is thus advantageous for issues of stability. We describe the further development of the selective homonuclear polarization transfer sequence, integrated with a rosette spectroscopic imaging trajectory and demonstrate this sequence for the detection of GABA in human brain at 7T.Methods
Fig. 1 demonstrates the pulse sequence for the j-refocused polarization transfer. As described (3), the base sequence is a double spin echo where J-coupling is induced in both echoes. A second 90 pulse is applied at the end of the first echo 90° shifted relative to the excitation pulse to induce homonuclear polarization transfer. This refocuses the J evolution compared to a single echo of equivalent total duration, albeit not completely for IS2 and IS3 spin systems. For selective polarization transfer editing, longitudinal suppression of a coupled partner (e.g., CHESS suppression of the 3.0ppm of C2 GABA) results in asymmetric polarization transfer, allowing transfer from 1.9ppm into the 3.0ppm position but without the overlapping 3.0ppm resonances. As the applied CHESS frequency can be adjusted, this method permits interrogation of arbitrary coupled spins.All studies were performed on a Siemens Magnetom 7T 8 channel pTx system with an 8x2 transceiver and very high order shim insert. With the 8x2 transceiver, the sequence was applied using two RF distributions, the intracerebral homogeneous (majority of sequence) and extracerebral ring (outer volume suppression of lipids). The use of the ring B1 distribution for OVS is sufficient for lipid suppression such that no additional in-plane selection or global suppression methods are needed. For the 3.0ppm GABA detection, a combined inversion recovery (simultaneous to pre-excitation water suppression) and an SLR optimized dual band CHESS pulse (420Hz passband, 300Hz stopband, 15ms) was used to suppress both the 3.0 and 1.65ppm positions (macromolecules which can transfer to 3ppm). The combined suppression approaches provides spectral baselines that are relatively less sensitive to T1 variation. For consideration of the 1.65ppm region, the dual band CHESS pulse was applied upfield to all 1H resonances (the same TE was used as for GABA). The rosette trajectory was used for encoding, being advantageous for spectroscopic imaging given its low gradient demands and high efficiency (4). The spectroscopic imaging was acquired with nominal resolution of 14x14x9mm, TR 3s/TE44ms with acquisition time of 7.5min.
Results
Fig. 2 shows spectra from a 7.5min spectroscopic imaging acquisition in a healthy control showing the consistency of the GABA resonance. The ratio of peak areas of GABA/tNAA is 0.040±0.013. With the efficiency of the acquisition determined from simulation to be 45%, and taking NAA at 10mM this generates an estimated GABA concentration of 1.3±0.43mM, which is within the range of reported values of in vivo GABA (1,2). For the detection of the macromolecule resonance, the efficiency of the polarization transfer will be affected by its coupling structure. For example, the 1.9ppm resonance is a quadruplet, and thus its intensity will be affected by both the C2 3.0 and C4 2.28ppm resonances. The efficiency of the transfer thus is less given the perturbation of the 2.28ppm from the dual band CHESS pulse that places 1.7-1.9ppm resonances in the stopband. Thus Fig. 2C shows a spectrum (summed from 6 voxels) to show the presence of the macromolecule spectrum that is positioned at 1.65ppm.Discussion
The high SNR at 7T as implemented with the ring B1 outer volume suppression with the selective polarization transfer acquisition allows fast spectroscopic imaging of GABA to be feasible, with acquisition times that rival those used for single voxel studies. As a single shot acquisition, it is robust to system instability and with the rosette encoding is very efficient for spatial encoding. Finally, the flexibility of the longitudinal editing strategy allows evaluation of arbitrary coupled spin partners which can be informative for experimental and spectral analysis.Acknowledgements
This work supported by National Institutes of Health, NINDS R01NS090417, R01EB024408, R01EB011639.References
1. Terpstra M Mag Res Med 2002 47(5):1009
2. Edden RA, Mag Res Med 2012 68(3):657
3. Pan JW, Mag Res Med 2013 69(2):310
4. Schirda C. Mag Res Med 2016 76(2):380.
Figures
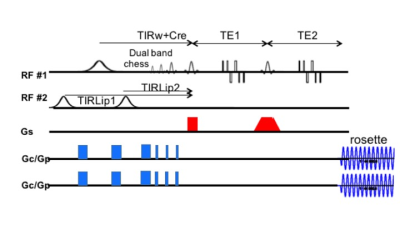
Fig. 1. Selective homonuclear polarization transfer pulse sequence with rosette trajectory encoding. There are two B1 distributions used, the primary homogeneous distribution used for localization, spin echoes and water suppression, the ring distribution used for outer volume suppression.
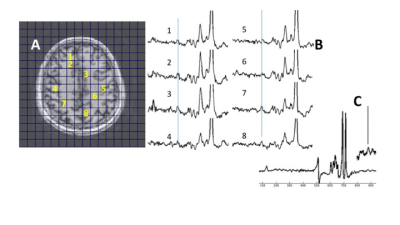
Fig. 2. (A,B) Scout, spectra from a healthy control showing spectra with corresponding locations. The line indicates the 3.0ppm position. (C) Editing acquisition targeting detection of the 1.6ppm region to identify the macromolecule resonance(s): 6 summed spectra shows the 1.65ppm macromolecule resonance.