2904
Potential benefits from Multi-Echo Single-Shot Spectroscopy with a combined fitting process1Department of Radiology and Biomedical Research, University of Bern, Bern, Switzerland
Synopsis
The benefits of multi-echo single-shot (MESS) spectroscopy are explored aiming at simultaneous determination of metabolite content and T2 times through simultaneous linear-combination model fitting of partially sampled echoes. Cramer-Rao lower bounds (CRLB) and Monte-Carlo simulations are used to judge this benefit. The novel scheme was compared with traditional multi-echo multi-shot and single-echo methods, exploring different TE settings for spectra of the major brain metabolites. Results indicate that MESS outperforms older methods for simultaneous determinations of T2s and concentrations, with improvements ranging at 20-30% for T2s and 30-50% for areas. However, for concentrations alone traditional single-echo sequences are more sensitive.
Introduction
Clinical MRS notoriously suffers from low SNR for basic diagnostic evaluation and lack of subject-specific relaxation times for full quantification. Various proposals have been made to arrive at better sensitivity for straight single-voxel MRS (improved multi-channel detection coils, higher B0 fields, large VOIs, fast acquisitions, machine-learning evaluations, etc.). Moreover, for additional comprehensive evaluations of concentrations and relaxation time (and macromolecular background) some methods based on combined evaluations of multiple different acquisitions (e.g. with different TE and/or TR or inversion times) have been proposed1-4, but are not in widespread use. However, it is also possible to obtain multi-echo data from a single acquisition, where a CPMG sequence can be used to prolong the range of high SNR to longer acquisition times - though at the expense of resolution of the spectra and at the expense of gaps in the acquired data where RF pulses and gradient crushers are applied5,6. Similarly, multi-TE data is in more widespread use in spectroscopic imaging, where‑just like in RARE‑multiple echo acquisitions can be used to cover k-space in different echo periods and speed the coverage of the full range7,8.In this work, we propose to explore theoretically the benefit of acquiring multi-TE data in single acquisitions to be used in a combined fitting process, where the half echo of the shortest TE is fitted with the full echo recorded for later TEs, including the extended tail of the last echo that provides resolution information for the whole echo train. Explorations are conducted by a Monte-Carlo approach, and mainly by calculation of Cramer-Rao Lower Bounds (CRLB) that rely on information of the interaction of model parameters and SNR to forecast optimal conditions without the need to actually acquire the data in vivo3. This work complements earlier work aimed at MRSI at longer echo times5.
Methods
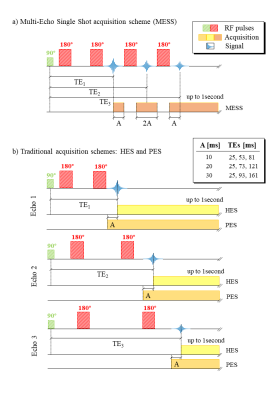
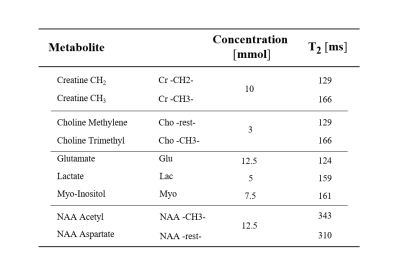
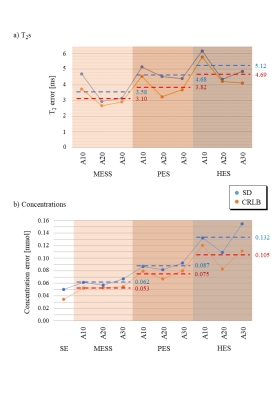
Multi-Echo Single Shot (MESS) spectroscopy as detailed in Fig. 1 was investigated in comparison to traditional single-echo (SE) and multi-echo multi-shot schemes. Spectra of a metabolite mixture9 specified in Table 1 were simulated at 3T using VESPA with Gaussian linewidths at 5Hz for a PRESS module with 3 echoes, as depicted in Fig. 2a. Ideal pulse rotations were assumed where pulse plus crusher gradients were assumed to occupy 4 and 8ms, respectively for 90° and 180° pulses. The residual time between echoes was used for signal acquisition characterized by a parameter A as defined in Fig 1a. Resulting TEs are also listed in Fig. 1. The first echo signal is acquired as a half-echo for A ms while the second and third echoes are acquired as partially sampled full echoes, where the last window lasts up to achieving a global 1 second acquisition length (sampling rate 4kHz). Conventional spectra as comparison were simulated accounting for 3 scenarios (Fig. 1b): separate acquisition of three single echoes with half echo sampling (HES) and partial-echo sampling (PES) at identical TEs as in MESS and also a single echo (SE) at the shortest TE (25ms). Monte-Carlo simulations (10 iterations/schedule) were evaluated with variable white Gaussian noise while preserving a comparable total experimental time for all schedules (i.e. $$$\sqrt(3)$$$ larger noise in HES and PES vs MESS and SE). Simultaneous 2D fitting and CRLB calculations were carried out in FitAID10. CRLB and standard deviation (SD) over results with different noise realizations are taken as measure for the achievable precision of the compared experiments.Results & Discussion
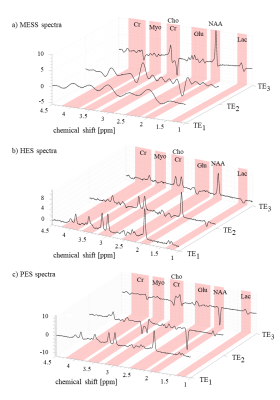
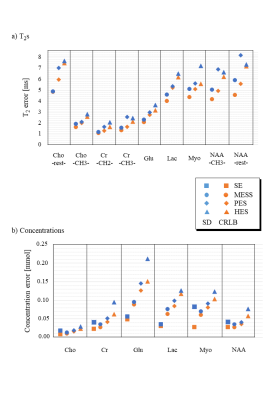
Fig. 2 illustrates instances of the simulated spectra according to the 3 different schedules (A=20ms). As expected, the short TE spectra in MESS (Fig. 2a) show very limited resolution (20 and 40ms acquisition length). MESS and PES spectra also show a linear phase according to the shifted start of data acquisition. However, all these frequency-domain issues are properly represented in the time-domain model. Figs 3 and 4 contain the main results. Fig. 3 shows the fit uncertainties in the form of CRLB and SD for the determined T2s and concentrations as function of experiment type averaged over the 3 different timings, while Fig. 4 presents them as averages over metabolites, but detailed according to TE settings. Precision of T2 determination is best for MESS, followed by PES and HES for all metabolites, and A20 and A30 seem better than the shorter TE combinations in MESS. For concentrations, the ranking is similar, except that the choice of TE does not have a large influence for the novel MESS scheme. However, for concentrations, it appears that in the cases investigated, simple short TE spectroscopy outperforms MESS.The current evaluations have clear limitations. Only a limited number of possible parameterizations (in particular ratio between T2 and T2* values5) have been tested; there is no experimental verification yet and the influence of the macromolecular signal has been ignored so far.
Conclusion
- A novel experimental scheme for optimal determination of concentrations and T2s for metabolites with complex spectral patterns has been tested in silico that combines short and long TE recordings from single acquisitions with simultaneous model fitting.
- The novel approach promises increased precision or inversely shorter experiment times compared to traditional approaches.
- Practical implementation and inclusion of the macromolecular signal are needed for final proof of superiority of the proposed scheme.
Acknowledgements
This work has been supported by the Marie-Sklodowska-Curie Grant ITN-39 237 (Inspire-Med).References
1. Kreis, R., Slotboom, J., Hofmann, L. & Boesch, C. Integrated data acquisition and processing to determine metabolite contents, relaxation times, and macromolecule baseline in single examinations of individual subjects. Magn. Reson. Med. 54, 761–768 (2005).
2. Just Kukurova, I. et al. Two-dimensional spectroscopic imaging with combined free induction decay and long-TE acquisition (FID echo spectroscopic imaging, FIDESI) for the detection of intramyocellular lipids in calf muscle at 7 T. NMR Biomed. 27, 980–987 (2014).
3. Bolliger, C. S., Boesch, C. & Kreis, R. On the use of Cramér-Rao minimum variance bounds for the design of magnetic resonance spectroscopy experiments. Neuroimage 83, 1031–1040 (2013).
4. An, L., Li, S. & Shen, J. Simultaneous determination of metabolite concentrations, T1 and T2 relaxation times. Magn. Reson. Med. 78, 2072–2081 (2017).
5. Kiefer, A. P., Govindaraju, V., Matson, G. B., Weiner, M. W. & Maudsley, A. A. Multiple-echo proton spectroscopic imaging using time domain parametric spectral analysis. Magn. Reson. Med. 39, 528–538 (1998).
6. Ronen, I., Ercan, E. & Webb, A. Rapid multi-echo measurement of brain metabolite T2 values at 7T using a single-shot spectroscopic Carr-Purcell-Meiboom-Gill sequence and prior information. NMR Biomed. 26, 1291–1298 (2013).
7. Duyn, J. H. & Moonen, C. T. W. Fast proton spectroscopic imaging of human brain using multiple spin‐echoes. Magn. Reson. Med. 30, 409–414 (1993).
8. Dydak, U. et al. Parallel spectroscopic imaging with spin-echo trains. Magn. Reson. Med. 50, 196–200 (2003).
9. Wyss, P. O. et al. In vivo estimation of transverse relaxation time constant (T2) of 17 human brain metabolites at 3T. Magn. Reson. Med. 80, 452–461 (2018).
10. Chong, D. G. Q., Kreis, R., Bolliger, C. S., Boesch, C. & Slotboom, J. Two-dimensional linear-combination model fitting of magnetic resonance spectra to define the macromolecule baseline using FiTAID, a Fitting Tool for Arrays of Interrelated Datasets. Magn. Reson. Mater. Physics, Biol. Med. 24, 147–164 (2011).
Figures