2900
Improved GABA editing and macromolecule suppression with adiabatic and highly selective Gaussian pulses at 7T1Centre d'Imagerie BioMédicale (CIBM), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Synopsis
The aim of the study is to improve the efficiency of GABA editing and macromolecule suppression at 7T. An asymmetric adiabatic pulse with broad inversion bandwidth was applied at 1.9 ppm to invert 1.5 – 1.9 ppm range and a narrow Gaussian pulse with 95 Hz of bandwidth (0.95 < Mz) was applied at 1.7 ppm to suppress macromolecule. In the GABA and lysine phantom tests, this scheme shows ± 9 Hz of signal loss threshold in comparison with highly selective inversion pulse without macromolecule suppression (± 6 Hz of threshold) and symmetric pulses (± 3 Hz of threshold).
Introduction
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter and its detection by 1H MRS is hampered by its low abundance and spectral overlap with other intensive metabolites such as creatine, and macromolecule (MM). MEGA is the most common editing method used for GABA editing. At 3T, due to limited spectral dispersion, MM is usually coedited with GABA leading to a GABA+ measurement at 3 ppm. Although MM suppression scheme was proposed by applying editing pulse symmetrically around 1.7 ppm (i.e. 1.9 and 1.5 ppm), this method is very sensitive to frequency drift during the acquisition. Another way to suppress co-edited MM at 3 ppm is to use inversion recovery, while this requires the knowledge of T1 relaxation and suffers from simultaneous GABA signal attenuation (1). Spectral dispersion largely increased at 7T, together with highly selective editing pulses, it may allow improved editing efficient with minimal MM contamination. MEGA was combined with sLASER localization to mitigate chemical shift displacement at 7T (2), whereas the short interpulse delay in sLASER limits the use of long editing pulses with high selectivity. MEGA-SPECIAL sequence has been proposed at 3T and it has the potential to incorporate long editing pulses. Therefore, to improve the chemical shift displacement and GABA editing efficient without coediting of MM at 7T, we implemented and compared three MEGA editing schemes in combination with semi-adiabatic SPECIAL localization.Materials and Methods
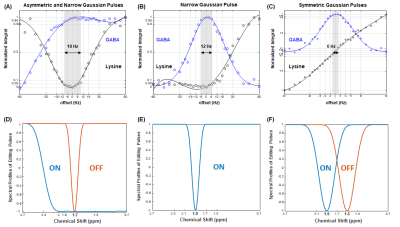
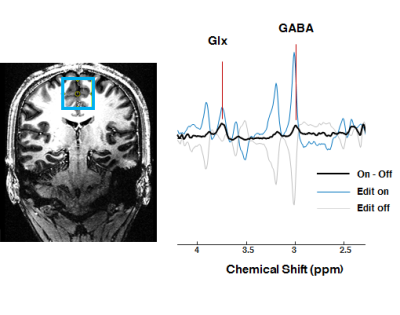
Three different frequency selective inversion pulses were generated for the study. A 20-ms asymmetric pulse has 136 Hz of transition bandwidth (-0.95 < Mz <0.95) on the one side and 500Hz of inversion bandwidth (Mz < -0.95), which consists of the first half of HS1 pulse and the second half of HS4 pulse. A 28-ms narrow Gaussian pulse has 95Hz of bandwidth (Mz < 0.95) and 7 Hz of inversion bandwidth (Mz < -0.95). A 15-ms long conventional Gaussian pulse has 140 Hz of FWHM and 20 Hz of inversion bandwidth (Mz < -0.95). All experiments were performed on a 7T/68cm scanner (Siemens Medical Solutions, Erlangen, Germany) with a 32-channel Nova coil. The semi-adiabatic MEGA-SPECIAL sequence (MEGA-sSPECIAL) was implemented as shown in Figure 1. 10 mM of GABA and lysine phantoms with TSP as an internal reference were prepared separately to test three MEGA-sSPECIAL schemes: 1) asymmetric (edit on at 1.9 ppm) and narrow Gaussian pulses (edit off at 1.7 ppm), 2) narrow Gaussian pulses (edit on at 1.9 ppm; edit off at 7.5 ppm), and 3) conventional Gaussian pulses for MM suppression (edit on at 1.9 ppm; edit off at 1.5 ppm). B0 shimming was performed using the first- and second-order shims with FAST(EST)MAP. The outer volume suppression and water suppression were interleaved prior to the sSPECIAL sequence. The following parameters were used: TR/TE = 6500/80 ms; VOI = 25×25×25 cm3; bandwidth = 4000 Hz; average = 40, number of data points = 2048. For the in vivo measurement, one volunteer was scanned using the scheme with asymmetric adiabatic and narrow Gaussian pulses. The receiver-channel recombination, B0 drift correction, and phase correction were performed using Matlab 2018a (The Mathworks, Natick, MA, USA). For in vitro test, GABA and lysine peaks are integrated between 2.85 ppm and 3.15 ppm.Results
Figure 2 illustrates the normalized GABA and lysine signal intensity at 3 ppm by three MEGA-sSPECIAL schemes: A) asymmetric pulse and narrow Gaussian pulse, B) narrow Gaussian pulse, and C) symmetric Gaussian pulses in order. Figure 2 (D-F) describe editing pulses used in MEGA on-off for each scheme accordingly. The frequency offset range was defined by ± 5 % of signal loss or increase of GABA and lysine peaks and marked in grey in Figure 2 (A-C). Figure 3 shows in vivo spectra acquired by MEGA-sSPECIAL with asymmetric adiabatic and narrow Gaussian pulses.Discussion
This study investigated three MEGA-sSPECIAL schemes with different types of editing pulses for the comparison of GABA editing and MM suppression efficiency at 7T. In combination with sSPECIAL localization, chemical shift displacement error is largely reduced to 6%/ppm in the refocusing direction. To incorporate selective editing pulses, TE was extended to 80 ms, therefore there is a 5.8 % signal reduction for GABA relative to that at TE of 68 ms assuming T2 of GABA is 63 ms (3). Phantom results suggested that scheme A (asymmetric adiabatic and narrow Gaussian pulses) is the least sensitive scheme for frequency drift with a bandwidth of 18 Hz, whereas scheme C (symmetric Gaussian pulses) is a highly sensitive method to frequency drift with a bandwidth of 6 Hz. The same narrow Gaussian pulse was used for both A) and B) but the measured bandwidths were different which may be attribute to the broader lysine peak at 1.7 ppm than GABA peak at 1.9 ppm (4).Conclusion
In conclusion, we proposed for the first time MEGA-sSPECIAL method with a combination of asymmetric adiabatic pulse and narrow Gaussian pulses, which improved the efficiency of GABA editing and MM suppression at 7T.Acknowledgements
We thank Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards and Jeantet Foundations.References
1. Moser P, Hingerl L, Strasser B, et al. Whole-slice mapping of GABA and GABA + at 7T via adiabatic MEGA-editing, real-time instability correction, and concentric circle readout. Neuroimage 2019;184:475–489 doi: 10.1016/j.neuroimage.2018.09.039.
2. Andreychenko A, Boer VO, Arteaga De Castro CS, Luijten PR, Klomp DWJ. Efficient spectral editing at 7 T: GABA detection with MEGA-sLASER. Magn. Reson. Med. 2012;68:1018–1025 doi: 10.1002/mrm.24131.
3. Intrapiromkul J, Zhu H, Cheng Y, Barker PB, Edden RAE. Determining the in vivo transverse relaxation time of GABA in the human brain at 7T. J. Magn. Reson. Imaging 2013;38:1224–1229 doi: 10.1002/jmri.23979.
4. Deelchand DK, Henry PG, Terpstra M. SPECIAL ISSUE RESEARCH ARTICLE MEGA ‐ PRESS of GABA + : Influences of acquisition parameters. 2019:1–9 doi: 10.1002/nbm.4199.
Figures