2899
A method to estimate GABA free from homocarnosine and macromolecular overlap through a combination of STEAM and J-difference editing sLASER at 7T1Biomedical Engineering, Columbia University, New York, NY, United States, 2Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 3Neurology, Yale University, New Haven, CT, United States, 4Radiology, Columbia University, New York, NY, United States
Synopsis
The aim of this work was to develop a novel method to assess accurately both the relative concentrations of homocarnosine as well as GABA free from overlapping creatine, homocarnosine and macromolecule signal. This was achieved via the combination of short echo time STEAM and MEGA-sLASER experiments at 7T. The metabolites GABA and homocarnosine were measured in 6 healthy control subjects, and in a single subject medicated with isoniazid. It was found that (16.6 ± 10.2)% of the supposed GABA signal originated from homocarnosine, and that isoniazid caused a significantly elevated concentration of GABA and homocarnosine in a single subject.
Introduction
The spectral peaks of GABA directly overlap with other metabolites, specifically the resonance at 3.01 ppm is obscured by both the methyl groups of creatine and phosphocreatine at 3.03 ppm, which have an approximately 20 times higher combined amplitude, as well as macromolecules at 3.01 ppm[1] and the GABA moiety of homocarnosine[2]. Thus for improved detection accuracy, GABA is typically measured by J-difference editing (JDE), which exploits intramolecular couplings[3] to resolve it from overlapping creatine/phosphocreatine. J-difference editing of GABA, however, cannot isolate the GABA signal from homocarnosine, which contains a GABA moiety with an extremely similar spectral signature in the region upfield from water[2]. The resulting GABA concentration therefore comprises both molecular GABA and contributions of homocarnosine’s GABA moiety and hence is referred to as GABAh.We applied a novel combination of inversion recovery-prepared JDE MEGA-sLASER[4]–[6] to estimate the concentration of GABA free from creatine/phosphocreatine and macromolecule overlap, and downfield short-TE STEAM to directly estimate the concentration of homocarnosine. The combination of these two measurements allows for the quantification of true GABA, free from homocarnosine or macromolecular overlap, as well as homocarnosine concentration.
Methods
A total of 7 subjects (ages 24-46, mean age years, 3 females) reporting themselves as healthy were scanned twice at Yale University’s 7T facility as part of a reproducibility study[7]. Two separate MRS experiments were performed. First, stimulated echo acquisition mode[8] (STEAM) was used with a voxel size of 2 x 2 x 2 cm3, TR = 3000 ms, TE = 10 ms, mixing time (TM) = 50 ms, 96 averages. Second, a data set was acquired using a JDE MEGA-sLASER sequence for GABAh editing with a voxel size 3 x 3 x 3 cm3 (larger voxel than STEAM due to the inherently low signal-to-noise ratio of GABAh), TR = 3000 ms, TE = 72 ms, 128 averages per editing condition. The optimal inversion time (TI) was experimentally determined to be 320 ms to minimize the signal from the 3.01 ppm MM resonance that directly overlaps with the upfield GABA and homocarnosine resonances.Processed spectra were fit with a least squares algorithm to the simulated basis sets made with MARSS[9] to extract relative concentrations with INSPECTOR[10]. All concentrations extracted from the linear combination modelling were calculated relative to creatine using the respective pulse sequence and corrected for T1 and T2 relaxation. The relative concentration of GABA (free from homocarnosine overlap) was calculated by subtracting the relative concentration of homocarnosine from GABAh. The significance of correlations were investigated between relative concentrations of GABAh and GABA with homocarnosine using an F-test with type I error, $$$\alpha = 0.05$$$. To compare whether the mean concentrations of the medicated Subject 2 differed from the rest of the subject population a two-sided Wilcoxon signed-rank test was performed with $$$\alpha = 0.05$$$.
Results and Discussion
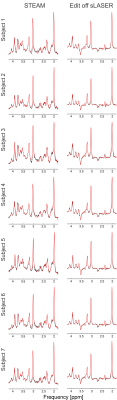
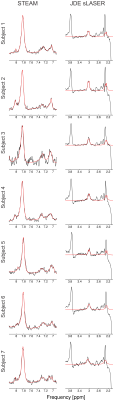
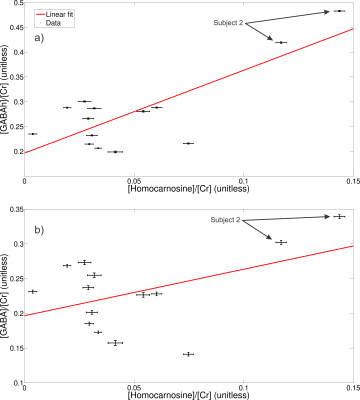
High spectral quality was observed for all upfield STEAM and edit off sLASER spectra for all subjects and trials (Figure 1). Readily identifiable homocarnosine peaks can be observed in most subjects, as well as signals from macromolecules at 7.10 and 7.30 ppm and a peak originating from NAA at 7.82 ppm (Figure 2). Noticeable elevation of homocarnosine of Subject 2 can be observed for both trials relative to the other 6 subjects. High signal-to-noise ratio GABAh peaks were observed for all subjects, and noticeable elevation of GABAh (relative to the glutamate + glutamine peak at 3.75 ppm) in Subject 2 compared to the controls can directly be observed (Figure 3).The relative concentration of homocarnosine to GABAh varied considerably in the subjects not treated with isoniazid (Table 1). There was found to be a significant correlation between the relative concentrations of GABAh and homocarnosine, P = 0.003, as seen in Figure 3. There was no significant correlation found between GABA and homocarnosine concentration (P = 0.221), despite them being metabolic partners. The fraction of GABAh signal at 3.01 ppm which actually originates from homocarnosine in healthy controls, obtained here using a linear combination modeling technique is (16.6 ± 10.2)% is in much better agreement with the expected value of 16.7%[12] than the previously obtained value using a peak-extraction method[11] of (29.4 ± 6.7)%. It is hypothesized the previously reported value is of substantially greater magnitude due to the direct overlap of homocarnosine with NAA and macromolecule resonances.
Comparing Subject 2 to the rest of the population cohort, there was a significant difference between mean relative concentration of homocarnosine (P = 0.022), GABAh (P = 0.022) and GABA (P = 0.022). There were no significant differences observed between the relative concentration of homocarnosine to GABA (P = 0.088) or GABAh (P = 0.088).
Conclusions
A novel combination of short-TE STEAM and JDE MEGA-sLASER were used to extract relative concentrations of homocarnosine, GABAh and GABA. It was demonstrated for the first time using linear combination modelling that a substantial contribution of GABA signal originates from homocarnosine. Additionally, it was demonstrated that for one subject on isoniazid significantly elevated GABA, GABAh and homocarnosine were observed, which has previously only been observed in post-mortem studies.Acknowledgements
No acknowledgement found.References
[1] K. L. Behar, D. L. Rothman, D. D. Spencer, and O. A. C. Petroff, “Analysis of Macromolecule Resonances in 1H NMR Spectra of Human Brain,” Magn Reson Med, vol. 32, pp. 294–302, 1994.
[2] V. Govindaraju, K. Young, and A. A. Maudsley, “Proton NMR chemical shifts and coupling constants for brain metabolites,” NMR Biomed, vol. 13, pp. 129–153, 2000.
[3] D. L. Rothman, O. A. C. Petroff, L. Kevin, and R. H. Mattson, “Localized 1H NMR measurements of y-aminobutyric acid in human brain in vivo,” Proc Natl Acad Sci USA, vol. 90, pp. 5662–5666, 1993.
[4] T. W. J. Scheenen, D. W. J. Klomp, J. P. Wijnen, and A. Heerschap, “Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses,” Magn Reson Med, vol. 59, no. 1, pp. 1–6, 2008.
[5] A. Andreychenko, D. W. J. Klomp, R. A. De Graaf, P. R. Luijten, and V. O. Boer, “In vivo GABA T2 determinaton with J-refocused echo time extension at 7 T,” NMR Biomed., vol. 26, no. 11, pp. 1596–1601, 2013.
[6] M. Mescher, H. Merkle, J. Kirsch, M. Garwood, and R. Gruetter, “Simultaneous in vivo spectral editing and water suppression,” NMR Biomed., vol. 11, no. 6, pp. 266–272, 1998.
[7] H. Prinsen, R. a de Graaf, G. F. Mason, D. Pelletier, and C. Juchem, “Reproducibility measurement of glutathione, GABA, and glutamate: Towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T.,” J Magn Reson Imag, vol. 45, no. 1, pp. 187–198, Jan. 2017.
[8] J. Frahm, K.-D. Merboldt, and W. Hänicke, “Localized Proton Spectroscopy using Stimulated Echoes,” J Magn Reson, vol. 72, no. 3, pp. 502–508, 1987.
[9] K. Landheer, K. M. Swanberg, and C. Juchem, “Magnetic Resonance Spectrum Simulator (MARSS), A Novel Software Package for Fast and Computationally Efficient Basis Set Simulation,” NMR Biomed, p. e4129, 2019.
[10] C. Juchem, “INSPECTOR - Magnetic Resonance Spectroscopy Software,” http://juchem.bme.columbia.edu/software-and-tools.
[11] O. A. C. Petroff, F. Hyder, R. H. Mattson, and D. L. Rothman, “Topiramate increases brain GABA, homocarnosine, and pyrrolidinone in patients with epilepsy,” Neurology, vol. 52, pp. 473–478, 1999.
[12] de Graaf RA. In Vivo NMR Spectroscopy: Principles and Techniques. 1998.
Figures