2898
Dual-voxel 1H MR spectroscopy in mouse brain at 14.1T with dynamic shim update (DSU) acquisition scheme
Hikari Yoshihara1, Nicolas Kunz2, Jean-Claude Martinou3, and Hongxia Lei2
1Laboratory for Metabolic Imaging, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2Animal Imaging and Technology Core (AIT), Center for Biomedical Imaging (CIBM), Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 3Faculty of Science, University of Geneva, Geneva, Switzerland
1Laboratory for Metabolic Imaging, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2Animal Imaging and Technology Core (AIT), Center for Biomedical Imaging (CIBM), Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 3Faculty of Science, University of Geneva, Geneva, Switzerland
Synopsis
Dynamic shimming updates with first- and second-order shims are essential for dual-voxel spectroscopy of mouse cortex and hippocampus at high magnetic field. We show that DSU is feasible for studying pharmacological effects in transgenic mice at 14.1 T.
INTRODUCTION
At high magnetic field, the challenges for multivoxel MRS in murine brains are substantially amplified. For instance, first- and second-order shim correction becomes essential to improve local field homogeneity. Thus, we aimed first to demonstrate the feasibility of acquiring in vivo high-field 1H MR spectra concurrently from both the mouse cortex and hippocampus at 14.1T using dynamic shim updates (DSU) on both shims without any supplementary hardware. The second aim was to apply the DSU approach to mice with glutamatergic neurons devoid of mitochondrial pyruvate carrier 1 (MPC1)1 and to their control littermates following i.p. injection of the GABAa receptor antagonist pentylenetetrazole (PTZ). Changes in the hippocampal neurochemical profile might occur, given the abundance of glutamatergic neurons there.METHODS
Animals :Eight male MPC1CAM2HO/TG mice bearing the modified MPC1 at glutamatergic neurons by the insertion of two flanking loxP sites that enable excision gene through Cre-mediated recombination were used for this study.1 Four were treated with tamoxifen dissolved in oil to generate the MPC1-/- genotype, and the other four received oil alone to remain MPC1+/+. On scanning days, all mice were anesthetized with 3% isoflurane mixed with 1:1 air and oxygen and their breathing rates were thereafter maintained in the range of 80-110bpm by adjusting the percentage of isoflurane (1-2%). 30min before the MRS measurements, mice were administered i.p. 20mg/kg pentylenetetrazole (PTZ), corresponding to half the dose that induced seizures in MPC1-/- mice in preliminary experiments (not shown). The head was fixed with two ear pieces and one bite bar, and rectal temperature was maintained at ~36ºC via circulating warm water via silicone tubes.
MR methods:
All experiments were performed in a horizontal 14.1T scanner, equipped with 400mT/m gradients (200μsec rise time) interfaced to a DirectDrive console (vnmrj, Agilent Inc.). Second-order shim coils are with maximum strengths of Z2 = 5.3 × 10−2mT/cm2, YZ = 1.2 × 10−1mT/cm2, XZ = 1.2 × 10−1mT/cm2, XY = 4.5 × 10−2mT/cm2 and X2Y2 = 4.2 × 10−2mT/cm2. A home-made quadrature surface coil (two geometrically decoupled 12mm-diameter loops) resonating at 600MHz was used for radio-frequency transmission and reception. Anatomical MR images were acquired using fast-spin-echo images (TEeffective/TE=50/4000ms, nt=8). Field homogeneities were optimized using FASTMAP2 on mouse unilateral cortex (1.8×0.8×2 mm3) and hippocampus (2.2×1.2×1.5 mm3), and the parameter sets saved separately. Localized 1H MRS (TE/TR=2.8/4000ms) was acquired using SPECIAL3. The optimized spectral parameters, including excitation, water suppression, outer volume suppression and the number of averages per scan (e.g. 16 averages per scan), for cortex and hippocampus were stored separately. The spectral measurement windows were between 0.5-1 hour after the PTZ-injection.
Dynamic Shim Update (DSU):
With the corresponding shims and the optimized spectral parameters, the dynamic shim update (DSU) acquisition scheme (Figure 1) was applied to both mouse cortex (DSUcortex) and hippocampus (DSUhippocampus).
Data processing:
The unsuppressed water signals acquired from the same VOIs were used for quantification. Spectral data were then collected, frequency corrected and summed for further analysis with LCModel. All metabolites except macromolecules (Mac) in the basis set of the LCModel were simulated, i.e. alanine (Ala), ascorbate (Asc), aspartate (Asp), creatine (Cr), myo-inositol (Ins), γ-aminobutryric acid (GABA), glucose (Glc), glutamine (Gln), glutamate (Glu), glycine (Gly), glycerophosphocholine (GPC), glutathione (GSH), lactate (Lac), N-acetyl-aspartate (NAA), N-acetyl-aspartyl-glutamate (NAAG), phosphocholine (PCho), phosphocreatine (PCr), phosphorylethanolamine (PE), scyllo-inositol (Scyllo), and taurine (Tau).
RESULTS AND DISCUSSION
DSU both 1st- and 2nd-order shims without any extra hardware is feasible for dual-voxel spectroscopy at 14.1T. With updating the 2nd-order shims (Table 1), quality spectra were obtained in an interleaved fashion from two brain regions, i.e. cortex and hippocampus (Figure 2). The resulting linewidths and SNRs were 16±2Hz and 10±1.5 for cortex, and 11±2Hz and 16±1.2 for hippocampus, respectively. The quantified neurochemical profiles of both MPC1+/+ and MPC1-/- mice are summarized in Figure 3. Although the metabolic changes due to the low dose of PTZ under isoflurane anesthesia are not substantial and remain to be explored further, regional differences in Lac, Ins and Glu were noticeable (Figure 4). Since DSU enables interleaved acquisition of both cortex and hippocampus regions, the metabolite difference, e.g. lactate (Figure 4a), cannot be biased by differences in isoflurane exposure, a potentially confounding factor if the two regions were scanned sequentially. In addition, the DSU scheme offers concurrently studying two important and distinct brain regions in the very same animal upon a small dosage of the GABAa receptor antagonist, i.e. PTZ. A consistently lower GABA level was observed in the hippocampus of MPC1+/+ mice (unpaired student t-test p-vaule<0.05, Figure 3b). In summary, we show that DSU of interleaved high-field spectral acquisition is feasible for studying pharmacological effects in transgenic mice.Acknowledgements
This work was supported by the CIBM of the UNIL, UNIGE, HUG; CHUV, EPFL and Leenaards and Louis-Jeantet Foundations.References
1. Vanderperre B, Herzig S, Krznar P, et al. PLoS Genet. 2016;12:e1006056.
2. Gruetter R, Tkac I. Magn Reson Med. 2000;43:319‐323.
3. Mlynarik V, Gambarota G, Frenkel H, Gruetter R. Magn Reson Med. 2006;56:965‐970.
Figures

Figure 1.
Acquisition scheme for interleaved 1H MRS of mouse cortex and
hippocampus. 16 scans were averaged for each spectrum. The DSU acquisition
scheme
was repeated 10 times for a total scan time of 21.3
minutes.
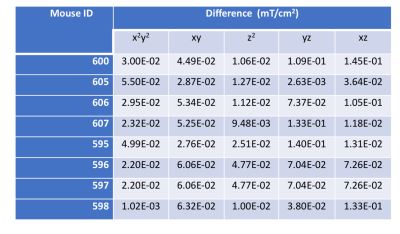
Table 1. Summary of 2nd-order shim (mT/cm2) differences between DSUcortex and DSUhippocampus of each mouse (ID).
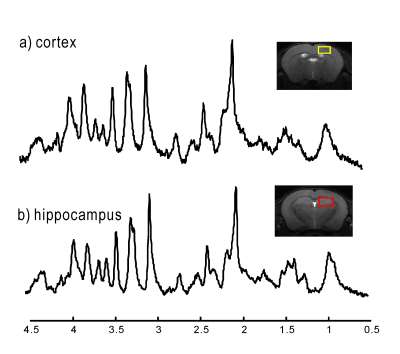
Figure 2.
Typical 1H MR spectra of cortex (a, yellow voxel) and hippocampus (b,
red voxel) of one mouse using DSU. Both spectra are averaged 160 scans and displayed
with 3Hz line broadening.
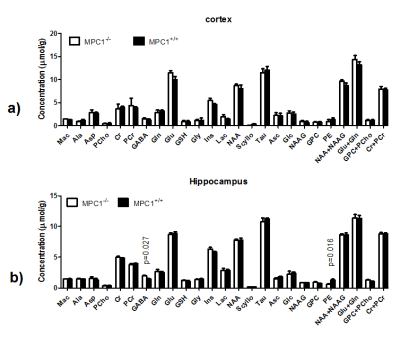
Figure 3. The neurochemical profiles of both cortex (a) and hippocampus (b) from MPC1+/+
and MPC1-/- after PTZ. Unpaired student t-test was carried on
metabolites and p<0.05 was reported. Error bars were standard errors
of means.
Abbreviations were listed in methods.
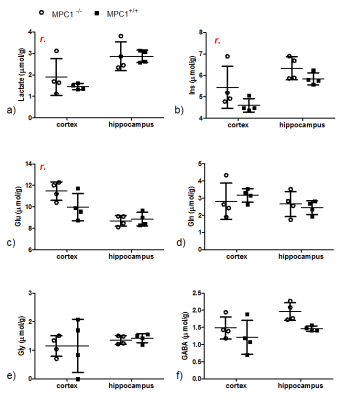
Figure 4.
Selected metabolites
of MPC1+/+ (solid squares) and MPC1-/- (open circles) mice
after the PTZ injection. Two-way ANOVA was performed on these metabolites with
two factors, region with matched subjects and genotype. “r” indicates
statistically significant (p<0.05) differences in metabolite levels between the two regions.