2894
Reproducibility of neurochemicals in sub-microlitre MRS1Core Facility Small Animal Imaging, Ulm University, Medical Center, ulm, Germany, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3German Center for Neurodegenerative Diseases (DZNE), ulm, Germany
Synopsis
Using sub-microlitre voxel size in magnetic resonance spectroscopy (MRS) is advantageous to investigate specific small brain region without any partial volume effect. The aim of this study was to determine the intra-individual coefficient-of-variation of neurochemical concentrations when using sub-microlitre MRS voxel measured during 3 consecutive days. This study was performed in adult mice at 11.7T. The intra-individual CV for the major and high concentration brain metabolites was ≤ 9% for MRS data collected in pre-clinically feasible scan times. This in turn shows that sub-microlitre can be used in pre-clinical application to detect subtle changes in neurochemical profile in diseased brain.
Purpose
Several pathological conditions affect only a small volume of the cortex (e.g. the motor cortex in amyotrophic lateral sclerosis (1) and its characterization in animal models is made impossible by the interference of normal, nearby cortical tissue. Therefore going to sub-microlitre voxel size with magnetic resonance spectroscopy (MRS) is advantageous in order to investigate specific small region without any partial volume effect. However, volume-of-interest (VOI) smaller than <1 µL will suffer from low signal-to-noise MR spectra even when a reasonable scan time is used. In order to use sub-microlitre MRS VOI in pre-clinical application, it is important to first evaluate the normal variation and test-retest reproducibility of neurochemical profile in normal healthy brains. Previous study in the rat brain (VOI of 63 µl) has reported the intra-individual coefficient-of-variation (CV) of high concentration metabolites e.g. phosphocreatine (PCr), creatine (Cr), glutamate (Glu), myo-inositol (Ins), N-acetyl aspartate (NAA), taurine (Tau) to be below 5% (2). In contrast, in mouse brain (VOI between 11 to 18 µL) the inter-individual CV for Cr+PCr (tCr) was <5% (3). Therefore, the goal of this study was to determine the test-retest reproducibility, as reflected by the intra-individual CV, of neurochemical concentrations measured in sub-microlitre VOI in adult mice measured during 3 consecutive days.Methods
Experiments were performed at a dedicated ultrahigh field 11.7T small animal system (117/16 USR BioSpec, AVANCE III, ParaVision 6.01, Bruker BioSpin, Ettlingen, Germany) equipped with a 9 cm inner diameter self-shielded gradient coil insert providing 750 mT/m maximal strength in 80 μs rise time. Cryogenically cooled 2-element phased-array transmit/receive coil was employed for excitation and signal reception. A home-built head restrainer was used to properly immobilize the animal's head during measurements, ensuring stability and reproducibility of the experimental setup. Volume-of-interests (VOI) were planned based on T1-weighted multi-slice FLASH (TR/TE = 193/5ms, flip angle 17.5°) images. Field homogeneity was adjusted for the investigated region using a field-map based approach (MAPSHIM). A short-echo-time LASER (Localization by Adiabatic SElective Refocusing) sequence (4) (TR/TE: 5000/16:75 ms: 10 kHz spectral width, 4096 data points and 386 averages) combined with VAPOR water suppression was used (5). Total acquisition time was 32 minutes and 10 seconds. In vivo 1H MR spectra were acquired from 0.729 mm3 (729 nl) volume located in the motor cortex of the three adult female C57BL/6. Single-shot data were frequency and phase corrected prior to summation (6). Unsuppressed water signal was used as an internal reference as well as for eddy current, zero-order and first-order phase correction. absolute metabolite concentrations were derived with LCModel (spectrum fitted from 0.5–4.2 ppm) (7). Metabolite concentrations with a Cramér–Rao lower bound (CRLB) ≤ 50% in at least half of the spectra in each brain region were used for statistical analysis. The sum of metabolites was reported when a high correlation existed between two metabolites (r<0.5, from Fisher matrix). The coefficient of variation (CV) of the metabolite concentrations were calculated to characterize intra-individual variability, as a measure of reproducibility.Results and Discussion
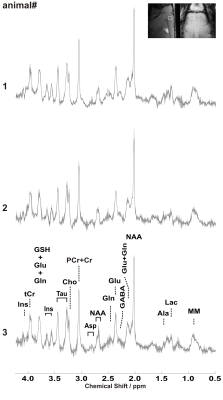
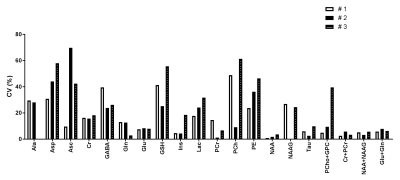
Sub-microlitre MRS data acquired from primary motor cortex in three adult mice during 3 consecutive days are shown in Figure 1. Well-resolved resonances of numerous cerebral metabolites signals can be observed. High quality spectra with reproducible spectral pattern were consistently obtained within animals and between scan days. The mean SNR of NAA for the 3 scans was 37±2 for animal #1, 31±6 for animal #2 and 33±2 for animal #3 respectively. Similarly, the mean water linewidth for the 3 scans was 13.3±0.3 Hz, 14.3±0.4 Hz and 13.6±1.2 Hz for animal #1, #2 and #3 respectively. These results in turn shows spectral reproducibility and VOI placement between animals for each scan. The intra-individual CV of the estimated metabolite concentrations was calculated for the three sessions for each animal (Figure 2). The mean intra-individual CV for the major and high concentration peaks in the mouse brain e.g. glutamine (Gln), Glu , Glu+Gln (Glx), Ins, NAA, PCr, taurine (Tau), tCr and (NAA + N-acetylaspartylglutamate) tNAA was ≤ 9%. These values are consistent with previous CVs in the mouse brain where the VOI was at least 10 times larger [Tkac MRM 2003]. In contrast, the mean CV for choline containing compounds (tCho) was below 18% and was related to the high CV of tCho (~40%) from animal #3.Conclusion
Excellent reproducibility as reflected by the intra-individual CV was possible for several major metabolites when using sub-microlitre MRS VOI (i.e. 0.729 µL) in the mice brain. In conclusion, sub-microlitre voxel can be used in pre-clinical MRS application to detect subtle changes in neurochemical profile in diseased brain.Acknowledgements
NIH grants: P41 EB027061, P30 NS076408References
1. Kim J, Hughes EG, Shetty AS, Arlotta P, Goff LA , Bergles DE, Brown SP. Changes in the Excitability of Neocortical Neurons in a Mouse Model of Amyotrophic Lateral Sclerosis Are Not Specific to Corticospinal Neurons and Are Modulated by Advancing Disease. J Neurosci. 2017 Sep 13;37(37):9037-9053.
2. Pfeuffer J, Tkác I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time (1)H NMR spectra of the rat brain. J Magn Reson. 1999 Nov;141(1):104-20.
3. Tkác I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med. 2003 Jul;50(1):24-32.
4. Garwood, M. & DelaBarre, L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson 153, 155-177, (2001).
5. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med (1999) 41:649.
6. http://www.cmrr.umn.edu/downloads/mrspa
7. Provencher, Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed (2001) 14:260.
Figures