2893
Test-Retest Reproducibility of in vivo Cortical GABA and Glx Measurements with MEGA-PRESS: Comparing 32-Channel and 8-Channel Head Coils1Research Imaging Centre - MRI, Centre for Addiction and Mental Health, Toronto, ON, Canada, 2Psychiatry, University of Toronto, Toronto, ON, Canada
Synopsis
Higher-numbered phased-array coils have been associated with significant increases in SNR. For this gain, one would expect more accurate and precise MRS measurements for a regular PRESS sequence processed with a fully automated program such as LCModel. However, the effects of increased SNR on a J-edited technique such as MEGA-PRESS for GABA measurements are not as straight-forward. In this study, we wish to compare the reproducibility of GABA+ and Glx measurements from an 8-channel and a 32-channel head coil in two cortical regions of interest: Anterior Cingulate Cortex (ACC) and Left-Dorsolateral Prefrontal Cortex (Lt-DLPFC).
Introduction
Higher-numbered phased-array coils have been associated with a significant increases in SNR, particularly in regions closer to the coil1. Several studies have compared the effects of number of arrays on structural, diffusion, and functional imaging with mixed results2,3,4.The effect of number of arrays on MRS results would be expected to be dependent on the region of interest. For a large SNR gain, one would expect more accurate and precise MRS measurements for a regular PRESS sequence processed with a fully automated program such as LCmodel5. However, the effects of increased SNR on a J-edited technique such as MEGA-PRESS for GABA measurements are not as straight-forward. The accuracy of editing techniques depends on the efficacy of the editing pulse, dependent on field homogeneity and not SNR. Furthermore, the method used to fit the difference spectra can have an effect on the reproducibility as well.In this study, we wish to assess the benefit of going from an 8-channel to 32-channel head coil for two cortical regions of interest (ROI) which are expected to be most influenced by coil choice. In particular, we want to quantify the reproducibility of the MEGA-PRESS method for GABA+ measurements in the Anterior Cingulate Cortex (ACC) and Left-Dorsolateral Prefrontal Cortex (Lt-DLPFC).
Methods
5 healthy participants (43±14 yrs; 1M and 4F) were recruited. All participants were scanned using a 3T GE MR750 scanner (GE Healthcare). Participants were first scanned using the 32-channel head coil (Nova Medical), 32HC. T1-weighted images were acquired for MRS voxel placement and for CSF-gray-white matter segmentation. We then scanned two consecutive (test/retest) MEGA-PRESS GABA+ scans in the ACC (Fig.1a) and Lt-DLPFC (Fig.1b). The 32HC was then replaced by the 8-channel coil (GE Healthcare), 8HC, and the same sequence of scans were repeated.MRS voxel-image co-registration was performed using Gannet3.05 and SPM126. The MRS voxel overlap between the two coils was calculated using FSL5.0-tools7.
Gannet3.0 and IDL-based program XsOs-NMR8 were used to fit the GABA+, Glx, and water peaks, as described in Fig.2a,b. The editing-OFF spectra were parsed and combined using an in-house script, which was then analyzed using LCModel9 (Fig.2c).
Reproducibility was computed as the percentage difference between the back-to-back measurements (m1,m2): %Diff=abs(m1-m2)/average(m1+m2). Measurements consisted of GABA+/H2O and Glx/H2O from the difference spectra and Glx from the LCmodel output for the edited-OFF spectra.
Results
MRS voxels were placed consistently across coils as given in Fig.1c. The one subject with an overlay of 76% was further investigated. Fractional tissue composition was consistent between the two coils for this subject. There were no significant differences in tissue composition between the 8HC and 32HC voxel placements for all subjects (p<0.00001) thus we did not do any correction for voxel composition to our metabolite measurements for purposes of reproducibility assessment.Results for the edited GABA+ and Glx measurements in the ACC are shown in Fig.3. In general, measurements resulting from XsOs-NMR were associated with lower %Diff for the 32HC. In the few cases where the %Diff was not reduced for the 32HC, the %Diff was small for both coils. In general, the reproducibility of Glx/H2O and GABA+/H2O were similar for XsOs-NMR on the 32HC (note change in y-axis). Measurements from Gannet3.0 were variable resulting in a lessened benefit to using the 32HC.
Results for the edited spectra GABA+ and Glx measurements in the Lt-DLPFC are shown in Fig.4. In this region results are more consistent across methods and metabolites: 32HC had lower %Diff with the exception of one case (GABA+/H2O for Subject 4) where the %Diff for the 32HC was more than double that in the 8HC. Inspection of the drift suggests that subject motion during the scan was a factor.
Fig.5 shows results for the water-scaled Glx measurements from the edited-OFF spectra processed with LCModel. Glx results are reproducible regardless of coil in the ACC but less so in the DLPFC (two subjects show %Diff>20% with the 32HC). Subject 1 had a poorer fit (%SD=15%) for the first measurement in the 32HC. Overall, Glx reproducibility was better when analyzing the editing-OFF data with LCModel compared to using the difference spectrum. Surprisingly, there were only slight increases in SNR with the 32HC vs 8HC. Although the 32HC linewidths were usually higher than the 8HC, it did not seem to affect the reproducibility of measurements.
Discussion
The reproducibility of GABA+ and Glx measurements were improved when the 32HC was used instead of the 8HC. Results depended on the fitting methods and slightly on the region (better consistency was seen in the Lt-DLPFC). XsOs-NMR with full manual fitting was favoured over Gannet3.0, at a cost of analysis time and possible operator bias.With Gannet3.0, there were outliers where metabolite peaks were overestimated when there are large bumps by the base of the peak. The fit range/parameters could be adjusted to account for these outliers.
The results of the editing-OFF spectra analyzed through LCModel generally were more reproducible compared to the difference spectrum fits, which were also noted by van Veenendall10. It would be interesting to also analyze the editing-OFF spectra using Gannet3.0 and XsOs-NMR to compare metabolites, such as NAA and Cr, across all three programs.
Acknowledgements
I would like to acknowledge Anusha Ravichandran M.R.T. at the RIC centre, whose expertise played a tremendous role setting up and acquiring the scans forthis study.
I would also like to thank the participants, as this study would not possible without their help.
References
1) Gruber, Bernhard, et al. “RF Coils: A Practical Guide for Nonphysicists: RF Coils.” Journal of Magnetic Resonance Imaging, vol. 48, no. 3, Sept. 2018, pp. 590–604. DOI.org (Crossref), doi:10.1002/jmri.26187.
2) Kaza, Evangelia, et al. “Comparison of a 32-Channel with a 12-Channel Head Coil: Are There Relevant Improvements for Functional Imaging?” Journal of Magnetic Resonance Imaging: JMRI, vol. 34, no. 1, July 2011, pp. 173–83. PubMed, doi:10.1002/jmri.22614.
3) Paolini, Marco, et al. “Resting-State Networks in Healthy Adult Subjects: A Comparison between a 32-Element and an 8-Element Phased Array Head Coil at 3.0 Tesla.” Acta Radiologica, vol. 56, no. 5, May 2015, pp. 605–13. DOI.org (Crossref), doi:10.1177/0284185114567703.
4) Panman, Jessica L., et al. “Bias Introduced by Multiple Head Coils in MRI Research: An 8 Channel and 32 Channel Coil Comparison.” Frontiers in Neuroscience, vol. 13, July 2019, p. 729. DOI.org (Crossref), doi:10.3389/fnins.2019.00729.
5) Edden, Richard A. E., et al. “Gannet: A Batch-Processing Tool for the Quantitative Analysis of Gamma-Aminobutyric Acid-Edited MR Spectroscopy Spectra: Gannet: GABA Analysis Toolkit.” Journal of Magnetic Resonance Imaging, vol. 40, no. 6, Dec. 2014, pp. 1445–52. DOI.org (Crossref), doi:10.1002/jmri.24478.
6) Ashburner, John, and Karl J. Friston. “Unified Segmentation.” NeuroImage, vol. 26, no. 3, July 2005, pp. 839–51. DOI.org (Crossref), doi:10.1016/j.neuroimage.2005.02.018.
7) Jenkinson, Mark, et al. “FSL.” NeuroImage, vol. 62, no. 2, Aug. 2012, pp. 782–90. DOI.org (Crossref), doi:10.1016/j.neuroimage.2011.09.015.
8) Shungu, Dikoma C., et al. “Brain γ-Aminobutyric Acid (GABA) Detection in Vivo with the J -Editing 1 H MRS Technique: A Comprehensive Methodological Evaluation of Sensitivity Enhancement, Macromolecule Contamination and Test-Retest Reliability: Evaluation of Brain GABA Detection with the J -Editing Technique.” NMR in Biomedicine, vol. 29, no. 7, July 2016, pp. 932–42. DOI.org (Crossref), doi:10.1002/nbm.3539.
9) Provencher, Stephen W. “Estimation of Metabolite Concentrations from Localizedin Vivo Proton NMR Spectra.” Magnetic Resonance in Medicine, vol. 30, no. 6, Dec. 1993, pp. 672–79. DOI.org (Crossref), doi:10.1002/mrm.1910300604.
10) van Veenendaal, Tamar M., et al. “Glutamate Quantification by PRESS or MEGA-PRESS: Validation, Repeatability, and Concordance.” Magnetic Resonance Imaging, vol. 48, May 2018, pp. 107–14. DOI.org (Crossref), doi:10.1016/j.mri.2017.12.029.
Figures
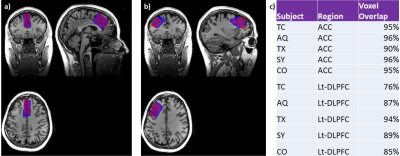
Figure 1: MRS voxel overlays of the 32HC and 8HC in the ACC (a) and Lt-DLPFC (b). The 8HC scans were registered onto the 32HC's T1-weighted image as reference. The overlay match% are displayed in (c). Both ROIs had the same acquisition parameters: TE/TR = 68/1500 ms; spectral width = 5000 Hz; number of points = 4096; number of averages = 192; NEX = 8; voxel size = 20 x 30 x 40, scan time = 5 min 12 s. The frequencies of the editing RF pulses were centered to suppress the C3 resonance of GABA (1.9 ppm) during the editing-ON acquisition and 7.5 ppm in the editing-OFF acquisition.
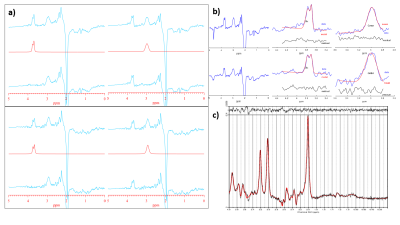
Figure 2: Outputs from XsOs-NMR (a), Gannet3.0 (b) -data from 32HC (top) and 8HC (bottom). The editing-OFF spectra were parsed and combined using an in-house script, which was then analyzed using LCModel (c). In XsOs-NMR, data was zero padded to 8192 points, an exponential 3Hz line broadening applied, and the metabolite peaks were fitted using pseudo-Voight lineshapes. With Gannet3.0, data was zero-padded to 32K points and an exponential line broadening of 3 Hz was used. GABA and Glx were fitted using Gaussian lineshape, whereas a Lorentz-Gauss lineshape was used to fit the water peak.
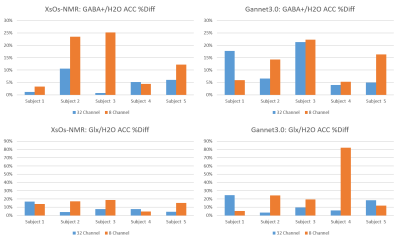
Figure 3: %Diff for 32HC and 8HC test-retest scans in the ACC. Results for GABA+/H2O were shown processed by XsOs-NMR (a) and Gannet3.0 (b). Results for Glx/H2O are shown in (c) and (d), processed by XsOs-NMR and Gannet, respectively.
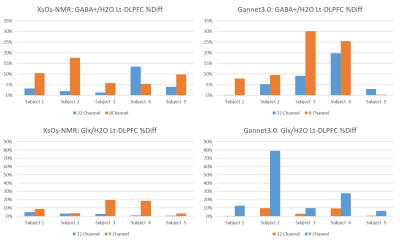
Figure 4: %Diff for 32HC and 8HC test-retest scans in the Lt-DLPFC. Results for GABA+/H2O were shown processed by XsOs-NMR (a) and Gannet3.0 (b). Results for Glx/H2O are shown in (c) and (d), processed by XsOs-NMR and Gannet, respectively.
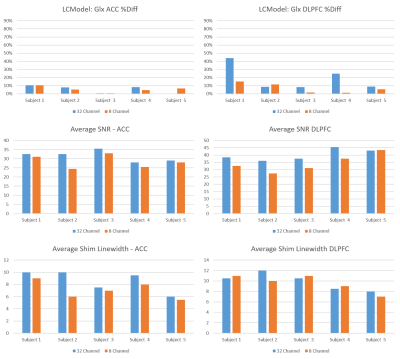
Figure 5: LCModel Glx results from the edited-OFF spectra for the 32HC and 8HC in the ACC (a) and Lt-DLPFC (b). Average SNR between the test-retest scans are displayed for the ACC (c) and Lt-DLPFC (d). Shim linewidths for the ACC and Lt-DLPFC are shown in (e) and (f), respectively. On average, going from 8HC to 32HC resulted in a SNR increase of 10% in the ACC and 15% Lt-DLPFC. Average shim linewidths in the ACC were 7.1Hz and 8.6Hz with the 8HC and 32HC, respectively. In the Lt-DLPFC, average shim linewidths were 9.6Hz and 9.9Hz with the 8HC and 32HC, respectively.