2889
Singlet state encoded magnetic resonance (SISTEM) spectroscopy1Medical faculty, MOIN CC, Kiel University, Kiel, Germany, 2Otto Diels Institute for Organic Chemistry, Kiel University, Kiel, Germany
Synopsis
MRS provides a non-invasive window into an in vivo biochemistry – basically a virtual biopsy. Here, we present two novel MRS sequences (SISTEM-1 and 2) and method to selectively prepare and detect the signal of endogenous metabolites. The long-lived signal of NAA-CH2 protons was isolated by the strong suppression of H2O and lipid signals using broad-band saturation or double-quantum filter. The frequency of the modulated NAA-CH2 signal was measured and associated with a pH value. Non-invasive measurement of pH value, imaging of drug distribution are only some of the possible applications.
Introduction
One of the most promising, yet least delivering MRI approach is maybe an in vivo magnetic resonance spectroscopy (MRS). MRS provides a non-invasive window into an in vivo biochemistry – basically a virtual biopsy. Still, its application in medical diagnostics is restricted: routine spectroscopy is typically limited to spatial, chemical and temporal resolutions of cubic centimetres, mM and minutes.There is a need for a new and simple MRS method that will provide full spectroscopic information about metabolites or other molecules in vivo.
Hyperpolarization techniques boost the signal of isotopically labelled metabolites and can provide requested spectroscopic information, however, it also requires a polarizer, administration of hyperpolarized substrate, specialized imaging sequences and an x-nuclei channel for the MRI system.
Here, we present a novel method to selectively prepare and detect the signal of endogenous metabolites.
Methods
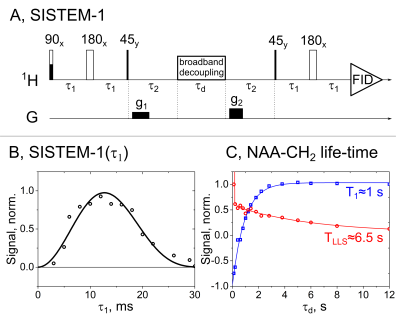
We use singlet-state encoded magnetic resonance (SISTEM) spectroscopy (fig. 1A and 3A) to selectively prepare the signal of metabolites for background free MRS. The pulse sequence utilizes the unique properties of long-lived singlet spin states (LLSs): (i) long live-time; (ii) two-spin quantum state; (iii) preservation of LLS under the action of broad-band decoupling. The double spin states of LLS are not destroyed by saturation pulses (fig. 1A) as other spin-orders are and can be isolated using double quantum filters (fig. 3A). The metabolite N-acetylalanine (NAA) was chosen as a test system.The method was tested on a 600 MHz spectrometer (Bruker Avance II) and 7 T MRI with a 30 cm bore (Bruker 7/30 ClinScan), equipped with a 1H transmit-receive volume coil with an inner diameter of 8 cm for excitation and a single-loop pick-up coil with an inner diameter of 0.9 cm for acquisition.
As a test system a solution of 10 mM NAA in 600 μl D2O (MS1) or in 300 μl D2O mixed with 300 μl dairy cream (30% fat concentration, MS2) was used for experiments with NMR and a solution of 100 mM NAA in 1500 μl H2O (MS3) was used for MRI studies.
Results
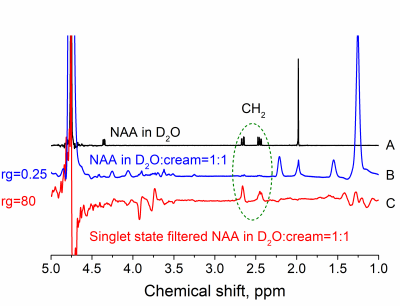
Two MRS sequences SISTEM-1,2 were successfully implemented on a high resolution 600 MHz NMR and preclinical 7 T MRI systems (fig. 1-3). The optimal value for SISTEM-1 (or SARKAR-II)1 pulse sequence (fig. 1A) for NAA-CH2 protons was found to be ca. 10 ms; sequence does not require precise settings (fig. 1B). The LLS was successfully populated and a lifetime of 6.5 s at 14.1 T and 2.5 kHz WALTZ-16 decoupling was obtained, that is almost 6 times longer than T1 ≈ 1 s (fig. 1C). This long lifetime of LLS and preservation under the action of hard RF-pulses allows saturating all other spins with a broad-band decoupling. This is a more efficient spectrum editing method than T1-weighted/differential approach.3Without any dedicated fat or water suppression, only a very little NAA signal can be detected by a pulse-acquisition experiment (fig. 2B). Using SISTEM-1, however, strong suppression of fat and H2O signal was achieved. This enabled an increase of the linear receiver gain, rg, by a factor of 320 (fig. 2C). It was realised in an inhomogeneous magnetic field (here: FWHM ca. 27 Hz).
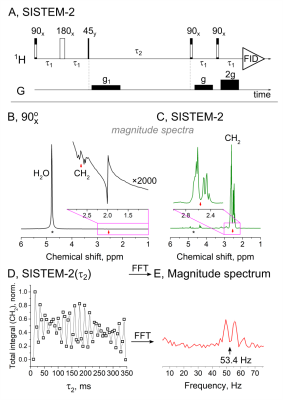
Due to limited allowed power deposition in the tissue, SISTEM-2 instead of SISTEM-1 sequence was used in MRI experiments. In SISTEM-2, the broadband decoupling and singlet-to-magnetization transfer stages are replaced by an OPSYd (fig. 3A)2. When applied, both water and NAA-CH3 signals were strongly suppressed, while the strong NAA-CH2 signal was observed (fig. 3B, C).
Zero-quantum coherences excited in SISTEM experiments evolve during the time period t2 with the frequency equal to chemical shift difference (fig. 3A,D). By acquisition of a series of SISTEM-2 spectra with different t2 and a consequent Fourier transformation, we were able to precisely determine the NAA-CH2 chemical shift difference of ca. 52.7+-0.4 Hz (fig. 3E). Due to severe magnetic field inhomogeneity of MRI this is impossible in a simple pulse-acquisition experiment.
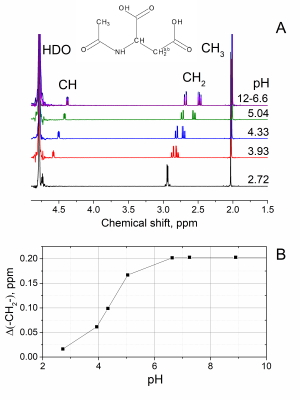
pH dependence of NAA spectrum was revealed by a high-resolution NMR (fig. 4). NAA-CH and NAA-CH2 protons are much more sensitive to pH variation than usually observed singlet of NAA-CH3.
Discussion
SISTEM allows separation of targeted long-lived signals by suppression of all the rest signals; the application may be to image the biodistribution of metabolites and drugs such as ethosuximide (Zarontin).pH measurement may be another application. The frequency of SISTEM modulated signal is equal to the chemical shift difference (fig. 3). The value of the chemical shift difference is a function of pH and for NAA-CH2 it changes from 0.2 ppm at pH 6 and above to 0.02 ppm at pH 3 (fig. 4). Hence, without a need for an external reference and correction of a magnetic field homogeneity and susceptibility, we were able to measure pH of model solutions.
Conclusion
We showed that LLS can be used to selectively prepare and measure the signals of coupled protons of chosen metabolites or drugs in the presence of water, inhomogeneous field and highly concentrated fatty solutions; before T1 contrast was used predominantly to improve spectral resolution and therefore quickly relaxing spins were not accessible. Non-invasive measurement of pH value, imaging of drug distribution are some of the possible applications.Acknowledgements
We acknowledge support by the Emmy Noether Program “metabolic and molecular MR” (HO 4604/2-2), the research training circle “materials for brain” (GRK 2154/1-2019), DFG - RFBR grant (HO 4604/3-1, № 19-53-12013), the Cluster of Excellence “Inflammation at Interfaces” (EXC306), BMBF grant e:Med Systems Medicine Initiative “Try-IBD” (01ZX1915C), the Cluster of Excellence “precision medicine in inflammation” (PMI 1267). Kiel University and the Medical Faculty are acknowledged for supporting the Molecular Imaging North Competence Center (MOIN CC) as a core facility for imaging in vivo. MOIN CC was founded by a grant from the European Regional Development Fund (ERDF) and the Zukunftsprogramm Wirtschaft of Schleswig-Holstein (Project no. 122-09-053).References
1. Sarkar, R., Vasos, P. R. & Bodenhausen, G. Singlet-State Exchange NMR Spectroscopy for the Study of Very Slow Dynamic Processes. J. Am. Chem. Soc. 129, 328–334 (2007).
2. Aguilar, J. A., Elliott, P. I. P., López-Serrano, J., Adams, R. W. & Duckett, S. B. Only para-hydrogen spectroscopy (OPSY), a technique for the selective observation of para-hydrogen enhanced NMR signals. Chem. Commun. 1183–1185 (2007).
3. Hövener, J.-B. et al. Whole-Brain N-Acetylaspartate MR Spectroscopic Quantification: Performance Comparison of Metabolite versus Lipid Nulling. Am. J. Neuroradiol. (2008).
Figures