2887
Improved MultiNet GRAPPA performance with semi-synthetic calibration data for accelerated 1H FID MRSI at 7T1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Max Planck Institute for Biological Cybernetics, Tuebingen, Germany
Synopsis
It has been shown that neural networks combined with variable k-space undersampling (MultiNet GRAPPA) is superior to a conventional GRAPPA reconstruction at 9.4T. Here, the feasibility of performing MultiNet GRAPPA for 1H FID-MRSI at 7T is investigated with and without novel modifications to the original acquisition/reconstruction scheme. In this study, it is shown that MultiNet GRAPPA is shown to be feasible for 1H MRSI acceleration at 7T with a new k-space undersampling scheme for higher signal-to-noise and increased map reliability and use of a novel technique to increase SNR retention using semi-synthetic calibration data without an increase in acquisition time.
Purpose
MR spectroscopic imaging (MRSI) can map metabolite distributions, but suffers from long acquisition times. To address this, acceleration techniques, like GRAPPA, have been applied to decrease acquisition times (1-3). It was recently proposed to improve upon the conventional GRAPPA reconstruction using multiple neural networks for MRSI reconstruction combined with variable k-space undersampling (MultiNet GRAPPA) (3). This approach was shown to be superior to a conventional 1H FID MRSI GRAPPA reconstruction at 9.4T. Here, the feasibility of performing MultiNet GRAPPA for 1H FID-MRSI at 7T is investigated with and without several novel modifications to the original acquisition/reconstruction scheme. In particular, we present (1) a new k-space undersampling scheme of 3.6x for higher signal-to-noise and increased map reliability and (2) a technique to increase SNR retention using semi-synthetic calibration data without an increase in acquisition time.Methods
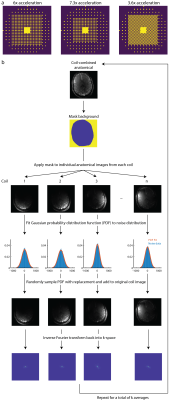
Figure 1a shows the k-space undersampling masks resulting in 6x, 7.3x, and 3.6x acceleration. Figure 1b shows a schematic of the method to artificially bolster the amount of training data. A Gaussian probability distribution is fit to the noise distribution in each coil taken from an anatomical image, randomly sampled, added to each voxel in the individual coil images, and inverse Fourier transformed back into k-space. This is repeated multiple times to form several semi-synthetic averages and used to train the neural networks that facilitate the GRAPPA reconstruction by predicting missing k-space points.Elliptically-sampled MRSI data were acquired in 3 healthy adults (2 male, age: 44.7 ± 14.7) on a Philips Achieva 7T scanner with a 32-channel receive head coil (Nova Medical). A 1H FID-MRSI sequence without lipid suppression (4, 5) was used as well as an optimized water suppression scheme (5). Data were acquired with a flip angle=32°, TR=300 ms, TE=1.02 ms, spectral bandwidth=4000 Hz, and 512 spectral points for a total acquisition time of 16 minutes. One dataset was acquired with a field-of-view of 192x192 mm2 at the level of the corpus callosum, while the other datasets were acquired with a field-of-view of 200x200 mm2 right above the level of the corpus callosum. All datasets had a slice thickness of 12 mm and used a 64x64 phase encoding matrix. A high resolution anatomical image at 4x4 times the resolution of the MRSI data was also acquired at the same slice position with the same dimensions as the MRSI data. A T1-FFE sequence was used with a flip angle=80°, a TR=37 ms, and a TE=4 ms.
The elliptically-sampled 1H-MRSI data was retrospectively undersampled which resulted in effective scan times of 4.4, 2.7, and 2.2 minutes for 3.6x, 6x, and 7.3x acceleration respectively. Data were reconstructed using MultiNet with two separate neural networks trained with 200 hidden layers for each level of undersampling. This resulted in 4 neural networks: 2-voxel cross-neighbor and 2-voxel adjacent-neighbor to fill the outermost k-space region and 1-voxel cross-neighbor, and 1-voxel adjacent neighbor to fill up to the fully-sampled center k-space region. For retrospective lipid removal, L1 regularization was applied to the reconstructed data (6). The performance of MultiNet GRAPPA was evaluated with several quantitative metrics: the signal-to-noise (SNR), structural similarity index (SSIM) (3) of the metabolite maps to the elliptically-sampled data, and Cramer-Rao Lower Bounds (CRLBs) of LCModel fits to the data. SNR was calculated as the ratio between the NAA peak over the root-mean-square of the noise from 10-12 ppm.
Results
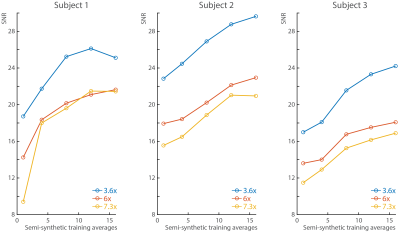
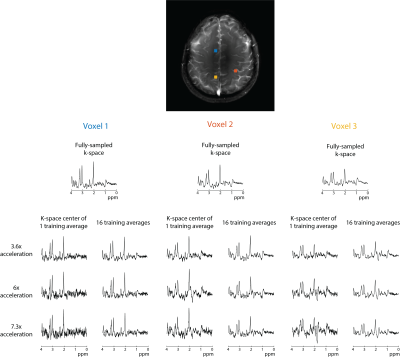
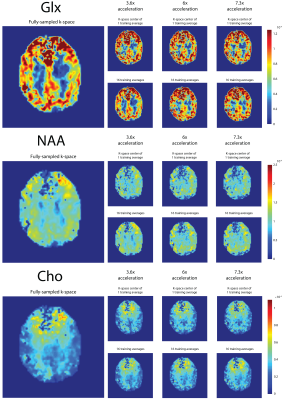
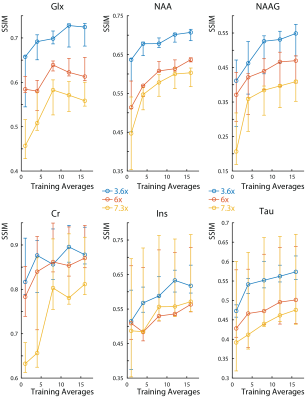
In all subjects, the SNR increases with the number of semi-synthetic anatomical averages used to train the neural network (Figure 2). In addition, the 3.6x accelerated data retains ~30% more SNR than the other accelerated data. The spectra reconstructed with 16 semi-synthetic averages retained 50.5% ± 15% more SNR than the spectra reconstructed with the center of k-space from one anatomical average as was originally proposed (3). This can be seen visually in figure 3 where the spectra reconstructed with 16 semi-synthetic anatomical averages are less noisy. These spectra are also visually similar to the spectra reconstructed from the fully-sampled data. Figure 4 shows metabolite maps for 3 major metabolites referenced to water. Visually, the maps reconstructed with 16 semi-synthetic averages are more similar to the maps generated from the fully-sampled dataset. In addition, the SSIM of the accelerated metabolite maps to the fully-sampled metabolite maps increases with more semi-synthetic training averages.Discussion
MultiNet GRAPPA is shown to be feasible for 1H MRSI acceleration at 7T with a new k-space undersampling scheme for 3.6x acceleration and semi-synthetic anatomical averages to improve neural network training. Furthermore, this method is simple to implement and doesn’t require additional hardware or software.The use of more semi-synthetic anatomical calibration data allows for improved neural network training, and consequently more accurate GRAPPA reconstructions. This results in improved SNR retention, and consequently, increased metabolite map similarity to the fully-sampled metabolite maps. While it is possible to instead acquire more anatomical data, this would result in increased acquisition times. For 16 averages, this would increase the acquisition time of the anatomical data from 10 seconds to 2.7 minutes. In addition, averages acquired in real time would be sensitive to head motion which would result in the neural networks learning motion between averages and therefore, less accurate GRAPPA reconstructions.
Acknowledgements
This work was funded by the Cancer Prevention and Research Institute of Texas (CPRIT) (Grant Number: RR180056) and European Union (Horizon2020, CDS-QUAMRI, Grant Number: 634541).References
1.
Hangel et al.,
NMR in Biomed 2015
2.
Moser et al.,
Magn. Reson. Med 2019
3.
Nassirpour et
al. NeuroImage 2018
4.
Henning et al,
NMR in Biomed 2009
5.
Nassirpour et
al. NeuroImage 2016
6. Bilgic et al. Magn. Reson. Med 2013
Figures