2886
Semi LASER Localized Echo Planar Total Correlated Spectroscopic Imaging1Radiological Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 2School of Health Sciences, Purdue University – West Lafayette, West Lafayette, IL, United States
Synopsis
We implemented a semi-LASER localized total correlated spectroscopic imaging (EP-TOCSI) sequence on a 3T MRI scanner. Compared to L-COSY the coherence transfer 900 RF pulse was replaced by a spin-lock module consisting of adiabatic train of RF pulses using MLEV4 phase cycling; also, multi-voxel encoding included EPI read-out combined with phase-encoding. After evaluating the performance of the 4D EP-TOCSI sequence using a corn oil phantom, we investigated calf muscle of 2 healthy and 1 type 2 diabetic subjects. There were relayed cross peaks in addition to COSY cross peaks conventionally recorded by L-COSY or EP-COSI.
Introduction:
Echo-planar correlated spectroscopic imaging (EP-COSI)1, which combines L-COSY2 with an echo-planar3 readout combined with phase-encoding gradients for correlated SI, facilitates improved detection of overlapping metabolites with increased spectral dispersion from multiple spatial regions. In skeletal muscle, EP-COSI has been used to differentiate saturated and unsaturated lipid components, allowing estimation of the degree of unsaturation4,5,6. Based on the same principle of COSY, total correlated spectroscopy (TOCSY)7 is another powerful technique that contains information about the directly scalar coupled spins coherence transfer. However, TOCSY can provide additional cross peaks for nuclei that are connected by a long chain of coupling and exhibits an in-phase magnetization transfer. This can be valuable, as in some cases metabolites might have pairs of neighboring spins with similar chemical shifts. In-vivo 1D spectral-editing TOCSY has been tried previously with both adiabatic8,9 and non-adiabatic mixing10. Different localized 2D version of TOCSY11,12 has been proposed and implemented in human brain. However, due to the complex nature of the sequence potential of TOCSY has not been fully exploited in-vivo as SAR associated with the requirement of a sustained train of RF pulses during TOCSY mixing remains the main issue. Here, we propose a novel version of localized 4D echo planar total correlated spectroscopic imaging (EP-TOCSI) and implemented in human calf muscle in-vivo at 3T. We used a short TE semi-LASER (sLASER) localization and introduce a novel mixing module containing multiple adiabatic B1-insensitive refocusing (BIREF-1) pulses13. We hypothesize that the proposed technique will detect relayed COSY resonances in human calf muscle.Materials and Methods:
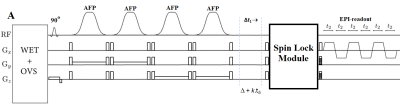
Fig.1 shows the schematic diagram of the EP-TOCSI sequence with sLASER localization. The spin-lock (SL) mixing module consists of train of four adiabatic BIREF pulses (duration of 6ms) with adiabatic half passage (AHP) tip-up/down pulses. The phases of the BIREF-1 pulses were prescribed according to MLEV-4 scheme14. TOCSY block does not contribute to the TE and total TE is nothing but the TE of the sLASER block. The time between the last AFP and the TOCSI block was incremented to introduce the t1 evolution. Global water suppression was performed using a WET15 scheme.To test the sequence, corn oil phantom data was acquired first. The sequence was further evaluated in the calf muscle of two healthy volunteers (age 37.5±6.4years) and one type 2 diabetic patients (T2DM) (65years). All data were collected on a 3T Prisma MRI scanner using a 15 channel knee ‘transmit/receive’ coil. The following parameters were used for acquiring the 4D EP-TOCSI data: TR/TE = 2s/29ms, voxel resolution=3.37cm3, 64 Δt1 increments, 512 bipolar echo pair, FOV= 24x24cm2, F1 and F2 bandwidths of 1250 Hz and 1190 Hz respectively. A non-water-suppressed scan with t1=1 was also recorded for eddy current correction and coil combination. For comparison, sLASER COSI with were also acquired with similar parameters as TOCSI, except TE=34 ms. Acquired data were extracted, reconstructed and post-processed16 with a library of custom MATLAB-based program.
Results:
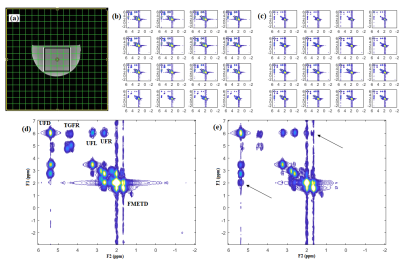
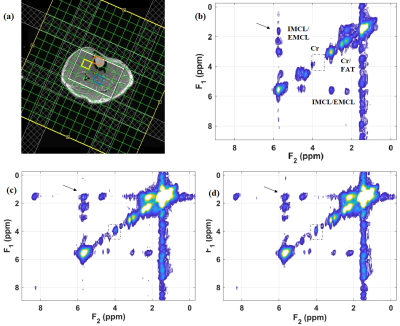
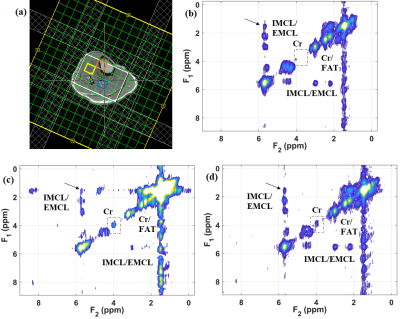
Fig. 2 shows our initial results in corn phantom showing extracted spectra recorded with sLASER EP-COSI and EP-TOCSI. Cross peaks of all lipid multiplets were well visible and separated from the main diagonal. Due to coherent transfer of in-phase magnetization in TOCSI the multiplet structure of each cross peak is more clearly apparent. Relayed cross peaks of intramyocellular (IMCL)/extramyocellular lipids (EMCL) are visible in TOCSI spectra which cannot be detected in EP-COSI. In vivo human calf muscle spectra from EP-TOCSI acquired in a 65 years-old T2DM patient can be seen in Fig. 3 from the bone marrow (b), tibias anterior (TA) (c) and the soleus muscle (SOL) (d). The absence of the water, Cr and Ch peaks in the marrow were expected and shows that the spatial information is preserved. It also preserves features such as the residual dipolar coupling of creatine in the tibialis anterior seen as a doublet, but remains a single peak in the soleus. Most importantly, the relayed cross peaks of IMCL/EMCL can still be observed. Fig. 4 shows the results from EP-TOCSI of a 42 years-old healthy volunteer showing spectra from bone marrow (b), TA (c) and SAL (d). Here also, relayed cross peak of IMCL/EMCL are visible demonstrating the reliability of the TOCSI technique.Discussion:
Combining semi-LASER localization and an echo planar readout, we have demonstrated feasibility of short TE EP-TOCSI using the corn oil phantom and human calf muscle. Although different versions of TOCSI have been implemented before for in-vivo brain study, this work represents the first demonstration of a robust in vivo TOCSI for calf muscle application. We demonstrated that TOCSI can uncover the hidden relayed cross peaks, particularly that of the unsaturated IMCL/EMCL in calf muscle which can play an important role in clinical application in diabetic/obese skeletal muscle studies for better estimation of degree of unsaturation. There are still few limitations to the work including long scan time, which will be addressed using acceleration in future studies.Conclusion:
In this study, a new SL module for TOCSI is proposed. While these initial results are promising, further optimization and validation with a larger pool of subjects is needed. We expect that the new developments presented in this work will facilitate in-vivo applications of TOCSI in clinical evaluations.Acknowledgements
This research was supported by grants from 1) NIH/NIBIB (5R21EB020883-02) and 2) NINDS 1R21-NS090956.References
1. Mayer D, Dreher W, Leibfritz D. Fast echo planar based correlation peak imaging: Demonstration on the rat brain in vivo. Magn Reson Med 2000;44(1):23-28.
2. Thomas MA, Yue K, Binesh N, et al. Localized two-dimensional shift correlated MR spectroscopy of human brain. Magn Reson Med 2001;46(1):58-67.
3. Posse S, DeCarli C, Le Bihan D. Three-dimensional echo-planar MR spectroscopic imaging at short echo times in the human brain. Radiology 1994;192(3):733-738.
4. Lipnick S, Verma G, Ramadan S, et al. Echo planar correlated spectroscopic imaging: Implementation and pilot evaluation in human calf in vivo. Magn Reson Med 2010;64(4):947–956.
5. Nagarajan R, Carpenter CL, Lee CC, et al. Assessment of Lipid and Metabolite Changes in Obese Calf Muscle Using Multi-Echo Echo-planar Correlated Spectroscopic Imaging. Sci Rep. 2017;7(1):17338.
6. Wilson NE, Burns BL, Iqbal Z, Thomas MA. Correlated spectroscopic imaging of calf muscle in three spatial dimensions using group sparse reconstruction of undersampled single and multichannel data. Magn Reson Med 2015;74(5):1199-1208.
7. Braunschweiler L, Ernst RR. Coherence Transfer by Isotropic Mixing: Application to Proton Correlation Spectroscopy. J Magn Reson 1983;53(3):521–528.
8. Marjanska M, Henry PG, Ugurbil K, Gruetter R. Editing through multiple bonds: Threonine detection. Magnetic Resonance in Medicine. 2008;59(2):245–251.
9. Marjanska M, Henry PG, Bolan PJ, et al. Uncovering hidden in vivo resonances using editing based on localized TOCSY. Magn Reson Med 2005;53(4):783-789.
10. Choi IY, Lee SP, Shen J. Selective homonuclear Hartmann-Hahn transfer method for in vivo spectral editing in the human brain. Magn Reson Med 2005;53(3):503–510.
11. Andronesi OC, Ramadan S, Mountford CE, Sorensen AG. Low-power adiabatic sequences for invivo localized two-dimensional chemical shift correlated MR spectroscopy. Magnetic Resonance in Medicine 2010; 64(6):1542–1556.
12. Andronesi OC, Gagoski BA, Adalsteinsson E, Sorensen AG. Correlation chemical shift imaging with low-power adiabatic pulses and constant-density spiral trajectories. NMR Biomed 2012;25(2):195–209.
13. Ugurbil K, Garwood M, Rath AR, Bendall MR. Amplitude and Frequency/Phase-Modulated Refocusing Pulses that Induce Plane Rotations Even in the Presence of Inhomogeneous B1 fields. Journal of Magnetic Resonance. 1988;78:472–497.
14. Levitt M, Freeman R, Frenkiel T. Broadband heteronuclear decoupling. J Magn Reson 1982;47:328–330.
15. Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B 1994;104(1):1–10.
16. Sarma MK, Huda A, Nagarajan R, et al. Multi-dimensional MR spectroscopy: towards a better understanding of hepatic encephalopathy. Metab Brain Dis 2011;26(3):173–184.
Figures