2879
ECLIPSE utilizing Gradient Modulated Offset-Independent Adiabaticity (GOIA) Pulses for Human Brain Proton MRSI1Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States
Synopsis
At high magnetic field strengths (≥3T), the utility of MRSI is impeded by challenges such as increased B1 field heterogeneity, increased RF power requirements for reduced chemical shift displacement errors (CSDE), and water/lipid contamination. In this work, the utilization of gradient modulated (GOIA) RF pulses, is investigated, with elliptical localization with pulsed second order fields (ECLIPSE) for MRSI. A 15 kHz GOIA pulse based ECLIPSE double-spin-echo MRSI sequence was developed. In vivo data demonstrate that the GOIA based MRSI method provides full-intensity metabolite spectra in edge of the brain voxels, and undetectable extracranial lipid signals within or outside the brain.
Introduction
Proton Magnetic Resonance Spectroscopic Imaging (MRSI) is a powerful technique that can map the metabolic profile in the human brain, non-invasively. At high magnetic field strengths (≥ 3T), MRSI is presented with a number of technical challenges including, lipid and water contamination, increased B1 field heterogeneity, and increased RF power deposition to reduce chemical shift displacement errors (CSDE). Elliptical localization with pulsed second order fields (ECLIPSE1) for MRSI has recently been demonstrated for robust (> 100-fold) lipid suppression, while providing high brain coverage relative to cubical localization and low power requirements compared to traditional 8-slice OVS2. The objectives of this work were to further improve ECLIPSE by reducing CSDE through the use of gradient-modulated GOIA3 pulses without significantly increasing RF power deposition.Methods
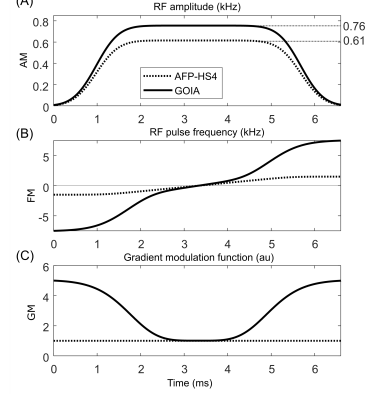
The GOIA RF pulse was derived from an AFP-HS4 pulse (Figure 1, dotted line) of 6.66 ms duration and 3.0 kHz bandwidth. Given the amplitude and gradient modulations, the GOIA frequency modulation was calculated for a 15 kHz bandwidth (Figure 1, solid line) as previously described3. In order to achieve 99.5% inversion efficiency, the GOIA RF pulse needed to be executed with 25% more amplitude (Figure 1A). All MR experiments were performed on a 4T magnet (Magnex Scientific Ltd.) interfaced to a Bruker Avance III HD spectrometer running ParaVision 6 (Bruker, Billerica, MA, USA). A within-brain B1+ optimized, 8-element Tx/Rx volume coil was used with a ± 30% and ± 60% B1+ variation within the brain and extracranial region, respectively.The ECLIPSE system1 is a home-built, unshielded gradient insert consisting of Z2, X2Y2, and XY second order spherical harmonic magnetic fields with efficiencies of 5.48, 2.58 and 2.76 Hz/cm2/A, respectively, driven by three independent 100A Techron 7780 current amplifiers (Techron, Elkhart, IN, USA) interfaced to a home-built multi-channel gradient controller4. The minimum and implemented rise times of the ECLIPSE magnetic fields are 660 and 1150 µs, respectively. A water reference MRSI was acquired and used for receiver amplitude and phase correction, as well as B0 eddy current compensation.
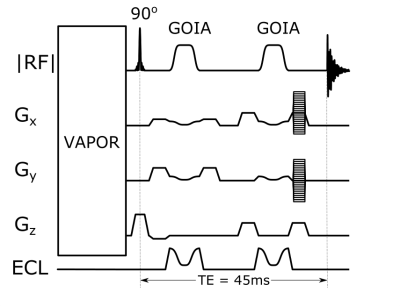
An ECLIPSE based adiabatic double-spin-echo sequence1 was implemented with GOIA RF pulses, for B1 insensitive inner volume selected MRSI (see Figure 2). The gradient modulation was applied to the higher-order Z2, X2Y2 and XY ECLIPSE gradients, as well as on the linear X and Y gradients needed for in-plane translations. GOIA modulation of the B0 field was implemented as a RF phase modulation. A seven-pulse VAPOR-style water suppression sequence was optimized using Gaussian pulses (10ms, 200Hz BW), as indicated in Figure 2. The MRSI phase encoding gradients were superimposed on the spoiler gradients (3 ms 20 mT/m), sampling a 17 x 21 matrix for a 1 mL nominal volume resolution.
Results
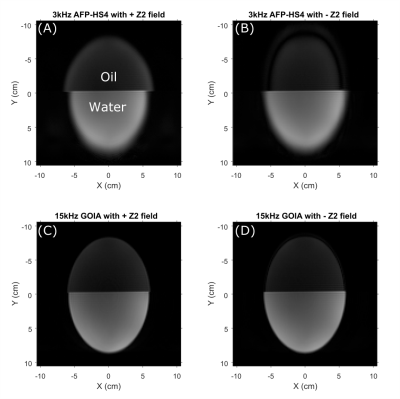
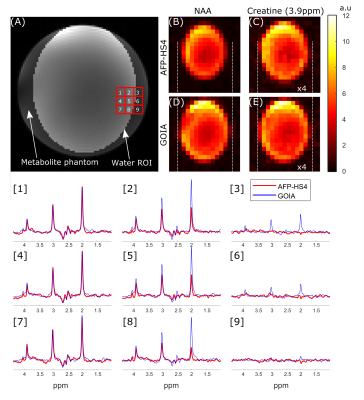
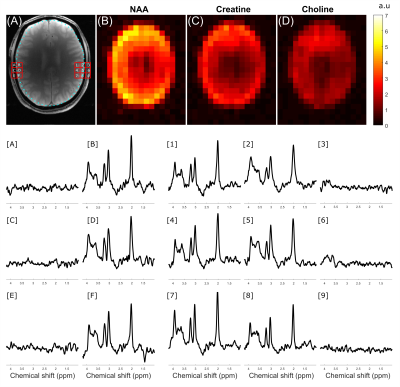
Figure 3 compares the CSD in a two-compartment water-vegetable oil phantom when the 3 kHz AFP-HS4 and 15kHz GOIA pulses were used with ECLIPSE localization. Considering water-fat chemical shift to be 600Hz at 4T, the CSD in (A-D) is 0.90cm, -0.90cm, 0.18cm, and -0.18cm respectively, which is in agreement with the apparent CSD in experimental data. A radially decreasing gradient field in the X-Y plane is generated for negative second order fields, and results in a smaller ROI for up-field lipid signals (see Figure 3B,D) relative to water. This convention was used for all subsequent MRSI acquisitions. Figure 4 compares the CSD for an elliptical ROI selection with ECLIPSE generated with AFP-HS4 and GOIA pulses in a metabolite phantom. The dotted lines that extend from (D) - (B) and (E) - (C) indicate the edge of the NAA methyl (2.01 ppm) and creatine methylene (3.93ppm) metabolic maps, respectively for the GOIA pulse ROI. The small water-creatine chemical shift difference leads to near-identical ROIs for both acquisitions. However, the circa 2.7 ppm water-NAA chemical shift difference leads to a significantly smaller ROI for the low-bandwidth AFP- HS4 pulse. When comparing voxel locations [2, 5, 8] the reduction in NAA intensity is evident, with the AFP-HS4 pulse, and is minimal with the GOIA based acquisition.Figure 5 illustrates an in vivo MRSI acquisition with GOIA pulses used for elliptical ROI selection with ECLIPSE. The elliptical ROI results in the water-NAA CSD to be 1.5mm and 1.9mm in the x and y direction at ROI edge voxels, respectively. Furthermore, the transition zone (Mz/M0 = 0.9 to -0.9) to bandwidth zone ratio for the GOIA (HS4) pulse is ~3%, which results in full intensity brain spectra along the ROI edge, and negligible perturbation of extracranial lipid resonances in the adjacent voxels as illustrated in Figure 5.
Conclusions
ECLIPSE-based IVS provides high-quality lipid suppression in MRSI. However, like all IVS methods it is sensitive to large CSDs during low bandwidth RF pulses. GOIA-ECLIPSE has been shown here to maintain excellent lipid suppression, while minimizing CSDs to 1.9 mm and providing high 2D brain coverage. The five-fold bandwidth improvement comes with a modest (~25%) increase in RF amplitude, making GOIA-ECLIPSE an attractive method for high-field MRSI.Acknowledgements
This research was supported by NIH grant R01- EB014861.References
[1] de Graaf RA, Brown PB, De Feyter HM, McIntyre S, Nixon TW. Elliptical localization with pulsed second-order fields (ECLIPSE) for robust lipid suppression in proton MRSI. NMR in biomedicine 2018;31(9):e3949.
[2] Henning A, Schar M, Schulte RF, Wilm B, Pruessmann KP, Boesiger P. SELOVS: brain MRSI localization based on highly selective T1- and B1- insensitive outer-volume suppression at 3T. Magn Reson Med 2008;59(1):40-51.
[3] Tannus A, Garwood M. Adiabatic pulses. NMR in biomedicine 1997;10(8):423–434.
[4] Nixon TW, McIntyre S, de Graaf RA. The design and implementation of a 64 channel arbitrary gradient waveform controller. Proc Int Soc Magn Reson Med. 2017;25:969.
[5] Tayari N, Heerschap A, Scheenen T, Kobus T. In vivo MR spectroscoping imaging of the prostate, from application to interpretation. Anal Biochem. 2017;529:158-170.
Figures