2877
Targeted fast spectroscopic imaging at 7T1Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Neurology, UPMC, Pittsburgh, PA, United States
Synopsis
The increased SNR at 7T and high efficiency of spatial encoding from fast readout trajectories means that substantially accelerated spectroscopic imaging acquisitions are practical. In this report, we implement targeted multi-slice Hadamard encoded cascaded and simultaneous acquisitions. With the cascaded rosette, minimal CSDE is incurred, generating 4slices in 8min12s. The J-refocused sequence achieves excellent CRLB in <2.3min for a single slice. With a B1 reduction strategy that allows multiple simultaneous Hadamard encoding, the J-refocused sequence is also demonstrated with 4slice acquisitions. As performed in brain tumor patients, the targeted Hadamard rosette is well tolerated and sensitive for detection of pathology.
Introduction
For extended volume spectroscopic imaging in the human brain, an acceptable clinically relevant goal is to acquire single and multiple axial slices at moderate to high resolution in-plane in under 10min. To achieve this goal, fast in-plane encoding and clean axial slice profiles (thereby minimizing partial volume overlap) are needed. The greater than 2.3-fold increase in SNR at 7T relative to 3T makes fast trajectories especially advantageous. We describe implementation of the rosette trajectory (1) with multi-slice capabilities at 7T for moderate spin echo and j-refocused sequences.Methods
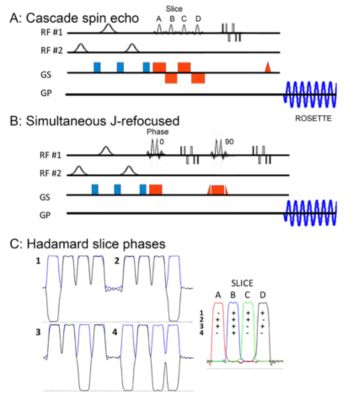
For multi-slice MRSI acquisitions at 7T, the slice encoding can be achieved either by conventional phase encoding, or by use of cascaded or simultaneous RF based Hadamard encoding. At low slice counts (e.g., 2 to 4), conventional phase encoding suffer from poor slice profiles and substantial slice profile bleed which is eliminated by Hadamard encoding (2). The RF-based Hadamard cascaded multi-slice acquisition maintains optimal chemical shift dispersion error (CSDE), but each slice has a slightly different TE. Due to B1 limitations, for the simultaneous Hadamard acquisition, the base pulse duration was doubled, and an encoding pattern was used to further reduce peak B1 requirements and reduce CSDE (Fig. 1). We demonstrate the cascaded and simultaneous multi-slice Hadamard rosette for moderate spin echo and j-refocused polarization transfer sequences. For the rosette trajectory, multiple shots of the circular rosette (1) are used with radial and angular oscillation frequencies of 625Hz with two temporal interleaves (spectral bandwidth of 2500Hz, Gmax 5.5mT/m; Smax 40.2mT/m/ms). The data were reconstructed as previously described (3) with matched scout images used to weight and phase the data for coil recombination.All studies were performed with a Siemens Magnetom Step 2.3 7T 8 channel system equipped with an Avanto body gradient coil, maximum slew rate of 200mT/m/ms, Gmax of 40mT/m and an 8x2 transceiver array (2 rows, 8coils/row, RRI Inc.). RF shimming is performed using two spatial B1+ distributions, targeting the whole brain (“homogeneous”) and extracerebral regions (“ring”) with the ring B1+ used for outer volume suppression (4). Non-iterative B0 shimming is performed using a very high order shim insert (VHOS, RRI Inc) providing 1st-4th order shims and two 5th order shims. We demonstrate this sequence in healthy controls and brain tumor patients.
Results
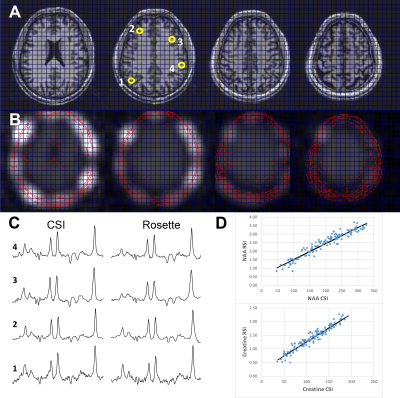
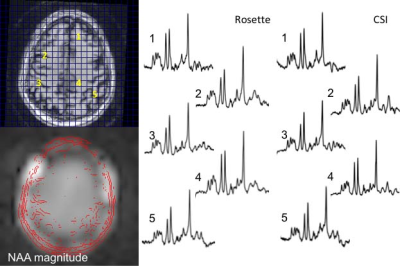
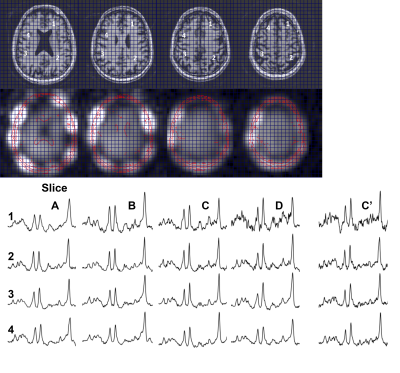
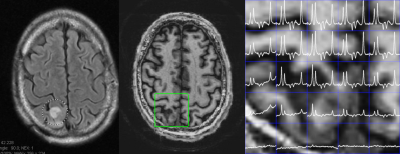
Fig. 2 compares data from a 4 slice cascaded moderate TE spin echo Hadamard acquisition, time matched (14.5min) with a single slice conventional phase encoded spectroscopic image (CSI). There is high consistency between these acquisitions, with a voxel-to-voxel analysis of Cr and NAA amplitudes showing a very significant correlation, R2=0.92. Spectral quality as assessed by the CRLBs for tNAA was not significantly different at 2.2±0.8 and 1.8±0.8 for the CSI and RSI respectively (2 subjects, n=140 voxels total). The J-refocused sequence is a phase sensitive double spin echo homonuclear polarization transfer acquisition. Fig. 3 shows that spectra from a 9mm thick 2.2min single slice J-refocused RSI acquisition have spectral quality that is comparable to a 9mm thick 14.5min phase encoded CSI acquisition of equivalent volume resolution. Despite the significant difference in acquisition time, LCM analysis shows no significant difference between the CRLB for glutamate, 4.8±1.7% (rosette) and 5.8±2.1% (CSI; n=2 subjects, ~130 pixels). Analyses of these data showed significant gray matter regression for the Glu/tNAA parameter for all subjects, intercept 0.49±0.15 and slope 0.58±0.06. To multi-slice the J-refocused acquisition requires the use of simultaneous (rather than cascaded) multi-slice excitation RF pulses. Using the reduced B1 Hadamard slice encoding for a 4-slice J-refocused acquisition, there is a chemical shift dispersion error in these data of 2.2mm between tNAA and Creatine (Fig. 4). The SNR is consistent with the 4fold longer acquisition time (8.8min) used for the multi-slice study in comparison with the matched 2.2min single slice acquisition.Fig. 5 shows data from a 4 slice Hadamard encoded moderate spin echo rosette acquisition in a 69yo tumor patient whose tumor was initially treated in 2009. Low grade progression was noted in 2017 and again with the present study identifying increased Ch/NAA at the gyral brain edge. This patient ultimately proceeded to additional chemotherapy.
Discussion
We have applied a rosette trajectory to achieve rapid MRSI acquisitions at 7T with <1cc resolution in single (2.2min) to four and eight slice acquisitions (8, 16min respectively). With two temporal interleaves as a 2D method, the rosette has very low gradient demands (Smax 40.2mT/m/ms, 20% of maximum to achieve a 9mm nominal isotropic resolution), is identical in gradient amplitude from shot to shot, and does not require additional eddy current corrections. We have shown that the SNR and CRLB values from the rosette are not significantly different from single slice conventional phase encoded acquisitions of equal duration, consistent with minimal loss due to the trajectory encoding. As applied in tumor patients, the rosette trajectory is well accepted by subjects and as shown, is sufficiently sensitive to demonstrate the pertinent pathology in neuro-oncology patients.Acknowledgements
This work supported by National Institutes of Health, NINDS R01NS090417, R01EB024408 and R01EB011639.References
1. Noll DC IEEE Med Imag 1997 16:372.
2. Gonen O Mag Res Med 1998 39:34
3. Schirda C Mag Res Med 79:2470 5.
4. Hetherington HP Mag Res Med 63:9
Figures