2864
A non-water-excitation MRS sequence with zero-echo time to investigate exchangeable moieties in the human brain at 3T1Departments of Radiology and Biomedical Research, University of Bern, Bern, Switzerland, 2Department of Biomedical Imaging and Image-guided Therapy, Medical University Vienna, High-field MR Center, Vienna, Austria
Synopsis
Most clinical MRS studies concentrate on the upfield part of the spectrum, neglecting the downfield region, since it is mainly composed of labile protons with intensities depending on the water suppression scheme. For reliable and sensitive quantification, a non-water-suppressed or even better a non-water-excitation (NWE) technique is required.
We introduce a non-echo technique based on ISIS localization for optimal detection of (moderately) fast exchanging protons at 3T with minimal signal loss due to T2 or exchange and with optimized water magnetization path for longitudinal relaxation enhancement.
Introduction
The downfield part of the human brain 1H MR spectrum is still poorly characterized due to two major obstacles - low concentration of implicated metabolites and the fact that most downfield resonances are affected by exchange with water, since water (pre-)saturation (WS) indirectly also saturates the magnetization of exchangeable moieties. Alternative techniques include metabolite cycling (MC)1,2 and water dephasing3, but both include periods where moderately fast exchange limits detection of labile protons (long TE, or times with opposite polarity of longitudinal water magnetization). As further alternative, non-water-excitation techniques were proposed, where the water magnetization is left untouched throughout the whole MRS sequence4,5,6. Persisting longitudinal water magnetization offers enhanced apparent T1 metabolite relaxation if the exchange is faster than ordinary T1-recovery (longitudinal relaxation enhancement (LRE)4).Motivated to observe as much of the fast exchanging proton pool as possible, we aim to probe different LRE schemes to achieve the shortest possible TE to prevent signal decay due to short T2 or exchange. Hence, the self-refocusing Band-selective Uniform-Response Pure-phase (BURP)7,8 pulses were introduced into an ISIS-based technique with additionally optimized LRE trajectory where positive longitudinal water magnetization (Mzw) was ensured after each ISIS cycle.
Methods
3D and 2D ISIS localization with Chemical-Shift-selective Excitation (I-CSE) sequences using GOIA inversion pulses6,9 were enhanced by replacing Gaussian chemical-selective excitation pulses by a BURP pulse. Furthermore, an additional water inversion pulse was introduced to precede the ISIS module to prevent negative Mzw at the start of the metabolite magnetization recovery period, as illustrated in Figure 1.Measurement parameters in short: 3T Prisma MR scanner (Siemens) with a multi-channel receive head coil; TR 3000, 850 or 650ms; spectral width 2500Hz, 128 averages with TE's of 10.2, 7.2, 8.4 or 0 ms, respectively, as indicated in the figures; down- and up-field metabolite-selective, plus water-selective scans; furthermore reference scans with conventional STEAM-MRS; supraventricular volume of interest (VOI) of 45x55x15mm3; data from 7 subjects presented. Signal processing including raw-data single-coil and single excitation averaging in MATLAB and evaluations in jMRUI10.
Results & Discussion
Results from introducing the BURP pulse are shown in Figure 2. TE dropped from 10.2 ms to 7.2 ms in the spin echoes with 2D ICSE. For 3D ICSE, BURP enables pulse-acquire spectra (TE=0, with acquisition delay of 0.2 ms vs. TE=8.4 ms as an echo). In both cases, the spectra with shorter TE clearly have a larger signal yield. To document the difference to water-suppressed MRS, Figure 3 illustrates results obtained with 3D ICSE at TE=0 with and without WS (CHESS with 3 pulses). The difference spectrum corresponds to signal loss induced by WS also affecting standard MRS.Figure 4 illustrates the effect of introducing a water-selective inversion pulse before the ISIS module such that Mzw remains along plus z for all steps of the ISIS cycle. This has been presented in Figure 4 for two TR's as average spectra from 6 subjects. The signal summation for localization remains unaffected since that concerns metabolite signal only. However, against original expectation, at both, short and long TR there is more signal in the spectra obtained without the additional pulse as visualized in the gray difference spectrum. The effect is smaller for the shorter TR, which can be well understood, when considering exchange in the localization part as well and not focusing on magnetization transfer (MT) during TR. The additional pulse does guarantee ideal conditions for LRE during TR but also leads to a state of disequilibrium between water and metabolites during the ISIS part. There, fast exchange then can lead to more signal loss through MT than what is gained by starting TR in a favorable condition. The short term effects dominate at long TR, hence bigger loss of signal in that case.
Moving the additional pulse from before the ISIS element to right before excitation ensures no imbalanced state between water and metabolites during the localization part. Hence, close to full signal for the fast-exchangeable moieties should be observable. This is confirmed in the preliminary results from a single subject presented in Figure 5. For short TR, a LRE effect is observed (more amide signal in the experiment with the additional pulse). To fully benefit from LRE, TR needs to be shortened to even shorter values, which may be though limited by SAR restrictions (even though the used GOIA pulses help to limit SAR). Signal gain by LRE is expected to be more effective at higher fields due to longer T1s but equal exchange times.
Conclusion
The proposed 3D BURP non-water-excitation sequence with signal acquisition immediately after the excitation pulse offers appreciable signal gain for fast exchanging and short T2 moieties.Introducing an additional water inversion pulse guarantees the proper magnetization state for LRE. Moving it directly before excitation gives promising results for LRE, where shortening TR renders the recorded signal more sensitive to exchange than T1 recovery.
Acknowledgements
This work is supported by the Swiss National Science Foundation (SNSF #320030‐175984).References
- Dreher W, et al. New method for the simultaneous detection of metabolites and water in localized in vivo 1H nuclear magnetic resonance spectroscopy. Magn Reson Med. 54:190–195, 2005
- Giapitzakis, et al. Metabolite-cycled STEAM with semi-LASER localization for MR spectroscopy of the human brain at 9.4T. Magn Reason Med 79: 1841-1850, 2018.
- Piotto M, et al. Gradient‐tailored excitation for single‐quantum NMR spectroscopy of aqueous solutions. J Biomol NMR. 2:661–665, 1992.
- Shemesh, et al. Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields, Nature Communications vol.5 4958, 2014.
- de Graff, et al. Detection of cerebral NAD+ in humans at 7T. Magn Reason Med 78:828-835, 2017
- Dziadosz, et al. Novel methods to record MR spectra in human brain without suppressing or exciting the water signal to investigate exchange-sensitive protons. Proc. Intl. Soc. Mag. Reason. Med., Montreal, Canada (2019) 0046
- Geen H, et al. Band‐selective radiofrequency pulses. J Magn Reson. 93:93–141, 1991.
- Bendel P, et al. Optimized 1H MRS and MRSI methods for the in vivo detection of boronophenylalanine. Magn Reson Med. 53:1166–1171, 2005.
- Bogner, et al. In Vivo 31P spectroscopy by fully
adiabatic extended image selected in vivo spectroscopy: A comparison between 3
T and 7 T, Magn Reson Med 66:923–930, 2011.
- Stefan, D. et al. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas. Sci. Technol. 20(10), 104035, 2009.
Figures
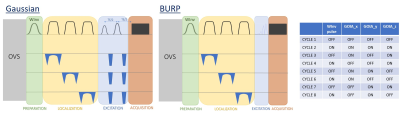
Fig 1 Diagram of the proposed sequences based on 3-dimensional ISIS localization with Chemical-Shift-selective Excitation (3D ICSE).
Left: using Gaussian pulses and echo formation; middle: using a self-refocusing BURP pulse for immediate acquisition.
Right: Pulse-cycling scheme based on 8 acquisitions.
The localization module is preceded by an optional additional water-selective inversion pulse (marked as WInv) to guarantee minimally disturbed water magnetization after the localization module. ISIS pulses moved as close as possible to the excitation module in all steps.
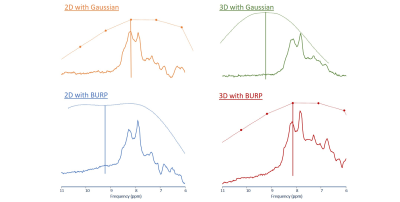
Fig 2 Illustration of the effect of introducing a self-refocusing pulse into ICSE sequences to reduce TE.
2D and 3D ICSE spectra acquired with Gauss or BURP excitation modules (with slice selective refocusing for 2D ICSE)6, including indication of the excitation profile of the respective pulses (dotted line) and the center frequency for excitation (solid vertical line: 4.5ppm from water for Gauss, 3.5ppm from water for BURP).
(2D ICSE: averaged from 2 subjects; TE 10.2ms (Gauss) and 7.2ms (BURP); 3D ICSE: single subject, TE = 8.4ms (Gauss) and 0ms (BURP); both with 3Hz broadening, TR 3s)
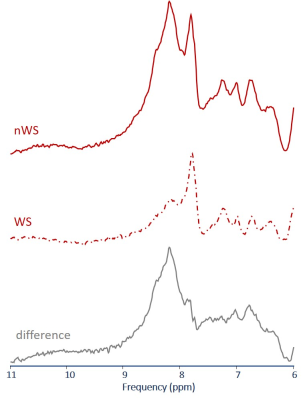
Fig 3 Illustration of the effect of water saturation and subsequent exchange of magnetization for NWE MRS.
Downfield spectra acquired with the non-echo 3D ICSE sequence without BURP pulse excitation without (nWS) and with (WS) water pre-saturation are displayed together with the difference spectrum containing spectral contributions of exchangeable protons.
(single subject, 3Hz Gaussian line broadening, TR=3000ms, 96 acquisition)
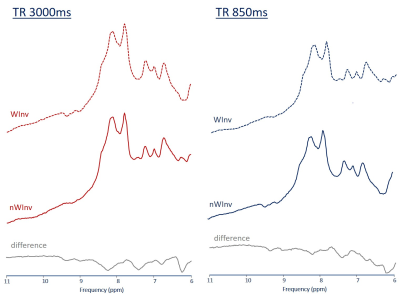
Fig 4 Illustration of the effect of an additional RF pulse to align post-acquisition water magnetization near the equilibrium.
Comparison of averaged spectra acquired with the non-echo 3D ICSE sequence with (WInv) and without (nWInv) additional water-selective inversion pulse. The difference spectra indicate that the extra pulse does not improve detection of exchangeable protons – likely because magnetization history immediately before excitation is unfavorable for fast exchanging protons.
(6 subjects, same acquisition time at both TRs: 128/448 acquisitions at 3s/0.85s TR)
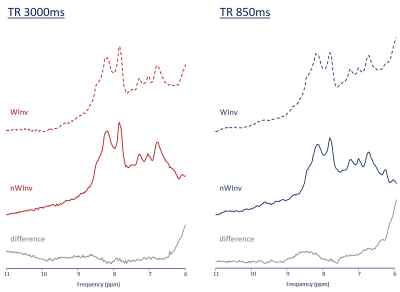
Fig 5 Illustration of effect of an additional water inversion pulse moved directly before excitation.
Spectra recorded with two TRs with 3D ICSE with (WInv) and without (nWInv) additional water-selective inversion pulse. The difference spectra (WInv - nWInv) imply that in this setup detection of labile protons is substantially improved at short TR documenting what has been reported as Longitudinal Relaxation Enhancement (LRE) at high field.
(single subject, excitation centered at 8.2 ppm; 3Hz broadening; acquisition times:as for Fig 4)