2863
UTE-SPECIAL for 3D Localization at an Echo Time of 4 ms on a Clinical 3 Tesla Scanner1Biomedical Engineering, Columbia University, New York, NY, United States, 2GE Healthcare, Berlin, Germany, 3Center for Magnetic Resonance Research and Radiology, University of Minnesota, Twin Cities, MN, United States, 4Radiology, Columbia University, New York, NY, United States
Synopsis
A previously developed magnetic resonance spectroscopy method is improved upon which is now able to obtain spectra with an echo time as short as 4 ms, while recovering the entirety of the available magnetization. This method obtains full 3D spatial localization through a 2D adiabatic inversion pulse which is cycled “on” and “off” every other repetition, in combination with a slice-selective excitation pulse. Both 1D and 2D spectra with an ultrashort echo time of 4 ms are demonstrated at 3T. High quality spectra were obtained for all experiments.
Introduction
It was recently recommended in a consensus paper to use the shortest TE achievable for general-purpose spectroscopy[1]. To obtain both a short echo time and full recovery of the signal a sequence referred to as SPin ECho, full Intensity Acquired Localized[2] (SPECIAL) was developed. Although SPECIAL provides a very short echo time it still employs a slice-selective refocusing pulse in a single spatial dimension, which introduces anomalous J-modulation[3], [4] and chemical shift displacement error (CSDE). As such SPECIAL-sLASER was developed which replaced the amplitude-modulated 180° refocusing pulse with an adiabatic refocusing pulse pair, but comes at the cost of a minimum TE of 19 ms at 3T[5].The proof-of-principle of a sequence we now refer to as Ultra short echo TimE SPECIAL (UTE-SPECIAL) was previously demonstrated[6], however it suffered from severe lipid contamination rendering the spectral quantification challenging. This abstract improves upon the previously developed sequence to obtain high quality spectra free of erroneous signal contributions. This was achieved by: 1) replacing the refocusing pulse by a hard pulse, thereby enabling the new ultrashort echo time of 4 ms, 2) using an optimized phase cycling scheme to reduce lipid contamination, 3) applying OVS in-between the preinversion pulse and excitation pulse to reduce out-of-voxel contamination. The measured spectra are now free from any obvious artefacts and can readily be quantified. Additionally, the method is extended to 2D spectroscopy and J-resolved spectra were acquired both in a phantom and a healthy volunteer.
Methods
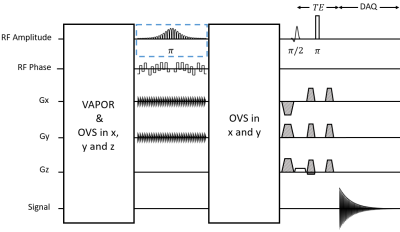
The UTE-SPECIAL sequence operates by employing a two-dimensional selective adiabatic inversion pulse[7] followed by a slice-selective excitation pulse and a hard refocusing pulse (Figure 1). The 2D inversion pulse consists of 30 spiral jinc subpulses with 5-turn spiral, and the amplitude and phase of the consecutive subpulses are modulated according to a hyperbolic secant envelope[8] with a total duration of 40 ms and a center frequency of 2.7 ppm. A two dimensional Bloch simulation of the adiabatic inversion pulse was performed to demonstrate the efficacy of the pulse. An optimized crusher and 32-step cogwheel phase cycling scheme[9] were designed with DOTCOPS[10], [11].All experiments were performed with a standard 48-channel head coil on a Signa Premier MRI system (GE Healthcare, WI, USA) at the New York State Psychiatric Institute (NYSPSI). All spectra were acquired with a TR of 2.0 s, 64 excitations and a cylindrical voxel with size of 5.8 x 5.8 x 2.0 cm3 (5.8 cm diameter in the inversion dimensions), and used VAPOR[12] for water suppression, as well as outer-volume suppression (OVS) for sideband suppression. Phantom experiments were performed on the GE MRS “braino” phantom[13].
A total of 5 male subjects were scanned, with multiple experiments being performed in some subjects. All subjects provided free and informed consent and all studies were approved by the IRB at Columbia University. Voxels were placed in either the frontal or parietal lobe. All subjects had spectra acquired with TE = 4 ms. Spectra were quantified with INSPECTOR[14] using basis sets simulated in MARS[15] using creatine as an internal reference assuming a concentration of 8.00 mmol/kg. J-resolved spectra were obtained both in the braino phantom and in vivo and were acquired by incrementing TE from 4 ms to 202 ms in 100 steps with 8 averages per echo time.
Results and Discussion
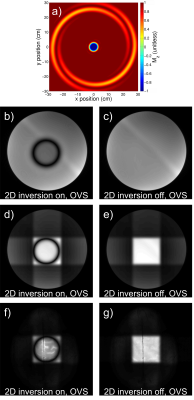
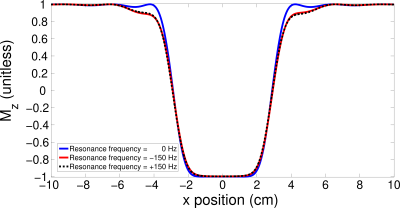
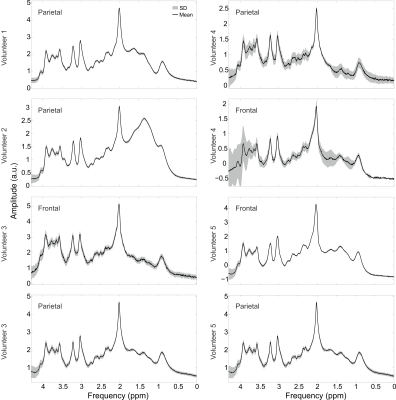
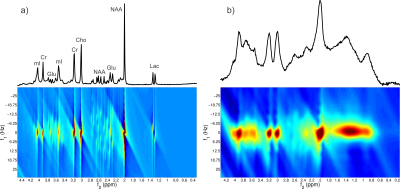
The 2D inversion pulse provides >99% inversion over 2.0 to 4.0 ppm and >97% inversion over 1.0 to 4.5 ppm. Bloch simulation of the 2D adiabatic inversion pulse demonstrates the high uniformity within the voxel with minimal sidebands (Figure 2 a), aside from large side bands at about 25 cm distance radially from the center of the voxel, which are outside the subject’s head for all volunteers. This was similarly demonstrated experimentally by imaging the voxel (Figure 2 b-g). Due to the subpulses being of very short duration (and thus high bandwidth) there is negligible CSDE in these two spatial dimensions (Figure 3).High spectral quality was demonstrated in all volunteers (Figure 4), however large macromolecule signals can be observed be observed in all volunteers, particularly for Experiment 2. This is not, however, believed to be artefactual, as subjects do not demonstrate any spurious echoes in the macromolecule region, as evident by the lack of increased deviation in this region. The mean (± standard deviation) concentrations were measured to be 3.10 ± 0.29 mmol/kg, 2.74 ± 0.34 mmol/kg, 1.59 ± 0.23 mmol/kg, 7.63 ± 0.82 mmol/kg, 10.56 ± 1.40 mmol/kg, and 4.87 ± 0.56 mmol/kg, for total choline, γ-aminobutyric acid, glutathione, glutamate + glutamine, N-acetylaspartic acid and myo-inositol, respectively. J-resolved spectra for both phantom (Figure 5 a) and in vivo (Figure 5 b) demonstrate the sequence’s lack of unresolved peaks,[16] particularly noticeable in the phantom lactate signal, due to the lack of slice-selective refocusing pulses.
Conclusions
A novel sequence was improved, referred to as UTE-SPECIAL, which now obtains high quality 3D localized spectra at the ultrashort echo time of 4 ms on a clinical 3T MR scanner, full intensity signal, adiabatic localization in two of the three spatial dimensions, no anomalous J-modulation and minimal CSDE. Quantification yielded results consistent with the literature.Acknowledgements
Special thanks to New York State Psychiatric Institute (NYSPI) and Dr. Feng Liu for their facilities and technical support and Martin Gajdošík, PhD, for fruitful discussions and input. This work was supported by the National Multiple Sclerosis Society (NMSS, RG-5319) and by NIH grant P41 EB027061.References
[1] M. Wilson et al., “Methodological consensus on clinical proton MRS of the brain: Review and recommendations,” Magn Reson Med, pp. 1–24, 2019.
[2] V. Mlynárik, G. Gambarota, H. Frenkel, and R. Gruetter, “Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition,” Magn Reson Med, vol. 56, no. 5, pp. 965–970, 2006.
[3] T. Lange, U. Dydak, T. P. L. Roberts, M. Bjeljac, and P. Boesiger, “Pitfalls in Lactate Measurements at 3T,” Am J Neuroradiol, vol. 27, pp. 895–901, 2006.
[4] K. Landheer, R. F. Schulte, M. S. Treacy, K. M. Swanberg, and C. Juchem, “Theoretical description of modern 1H in Vivo magnetic resonance spectroscopic pulse sequences,” JMRI, p. [Epub ahead of print], 2019.
[5] A. Fuchs, M. Luttje, P. Boesiger, and A. Henning, “SPECIAL semi-LASER with lipid artifact compensation for 1H MRS at 7 T,” Magn Reson Med, vol. 69, no. 3, pp. 603–612, 2013.
[6] Landheer K, R. Noeske, M. Garwoodd, and C. Juchem, “Double-Shot Semi-Adiabatic Localization for Ultra Short Echo Time Magnetic Resonance Spectroscopy,” 2019 Proc ISMRM, p. Abstract Number 1061.
[7] A. Jang, X. Wu, E. J. Auerbach, and M. Garwood, “Designing 3D Selective Adiabatic Radiofrequency Pulses With Single and Parallel Transmission,” Magn Reson Med, vol. 79, pp. 701–710, 2018.
[8] M. S. Silver, R. I. Joseph, C. N. Chen, V. J. Sank, and D. I. Hoult, “Selective population inversion in NMR,” Nature, vol. 310, pp. 681–683, 1984.
[9] M. H. Levitt, P. K. Madhu, and C. E. Hughes, “Cogwheel phase cycling,” J Magn Reson, vol. 155, no. 2, pp. 300–306, 2002.
[10] K. Landheer and C. Juchem, “Dephasing optimization through coherence order pathway selection (DOTCOPS) for improved crusher schemes in MR spectroscopy,” Magn Reson Med, vol. 81, pp. 2209–2222, 2019.
[11] K. Landheer and C. Juchem, “Simultaneous optimization of crusher and phase cycling schemes for magnetic resonance spectroscopy: an extension of dephasing optimization through coherence order pathway selection,” Magn Reson Med, p. [Epub ahead of print], 2019.
[12] I. Tkáč, Z. Starcuk, I. Y. Choi, and R. Gruetter, “In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time.,” Magn Reson Med, vol. 41, no. 4, pp. 649–56, 1999.
[13] T. Schirmer and D. P. Auer, “On the reliability of quantitative clinical magnetic resonance spectroscopy of the human brain,” NMR Biomed, vol. 13, pp. 28–36, 2000.
[14] H. Prinsen, R. a de Graaf, G. F. Mason, D. Pelletier, and C. Juchem, “Reproducibility measurement of glutathione, GABA, and glutamate: Towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T.,” J Magn Reson Imag, vol. 45, no. 1, pp. 187–198, Jan. 2017.
[15] K. Landheer, K. M. Swanberg, and C. Juchem, “Magnetic Resonance Spectrum Simulator (MARSS), A Novel Software Package for Fast and Computationally Efficient Basis Set Simulation,” NMR Biomed, p. e4129, 2019.
[16] P. B. Barker and R. A. E. Edden, “If J doesn’t evolve, it won’t J-resolve: J-PRESS with bandwidth-limited refocusing pulses,” Magn Reson Med, vol. 65, no. 6, pp. 1509–1514, 2011.
Figures