2841
Bilateral Sodium Magnetic Resonance Imaging of the Lower Extremity1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 3Rapid Biomedical GmbH, Rimpar, Germany, 4Mohn Medical Imaging and Visualization Centre, Department of Radiology, Haukeland University Hospital Helse Bergen, Bergen, Norway, 5General Electric Healthcare, Munich, Germany
Synopsis
We present a method for bilateral sodium magnetic resonance imaging (MRI) of the lower extremities. Sodium MRI can provide direct information on tissue biochemistry not available through standard proton MRI, and could therefore potentially assist in disease diagnosis. Our preliminary results demonstrate the application of large field-of-view sodium MRI to the musculoskeletal system for potential compositional assessment of multiple tissues in both legs including muscle, cartilage, synovium and arteries.
Introduction
Sodium magnetic resonance imaging (MRI) has the potential to improve the assessment of in-vivo tissue health by providing biochemical and potentially metabolic information.The sodium MR signal has been shown to correlate with glycosaminoglycan (GAG) concentration in articular cartilage1, intervertebral disks2,3, tendons4 and muscle. Negatively-charged GAGs in healthy tissue attract the naturally-abundant, positively-charged sodium ions. Therefore, sodium MRI could play an important in diagnosis and treatment monitoring of several diseases involving the musculoskeletal system, such as osteoarthritis5,6, muscular dystrophy7, tendinopathy4 or degenerative disk disease.
In this work we show preliminary results of bilateral sodium MRI of the lower extremities, including the calf muscles, knee joint tissues and thigh muscles. Future work could potentially allow simultaneous quantitative biochemical assessment of multiple tissues in the legs with one sodium MR measurement.
Methods
Both knees of three healthy volunteers (ages 27 to 34) were imaged simultaneously on a 3.0T MRI system (MR750 GE Healthcare, Waukesha, WI, USA) using a 50cm long and approximately 40cm inner diameter birdcage sodium transmit/receive coil (Rapid Biomedical, Rimpar, Germany). All imaging was performed with written informed consent provided by the volunteers and with approval of the local research ethics committee. Sodium MR images were acquired using a spoiled gradient echo 3D cones k-space trajectory with TR=100ms, TE=0.7ms, flip angle=70˚, field-of-view=48cm, voxel size=4x4x8mm3, averages=5, spiral interleaves=1402, Bandwidth=166, scan time=11:41min.Additionally, two low resolution sodium images were acquired with flip angles of 40˚ and 80˚, 196 spiral interleaves, and scan time = 24s per flip angle. From this data, flip angle (B1) maps were calculated as the ratio of actual flip angle to nominal flip angle. Anatomical proton images were acquired using the standard proton body coil and a 3D gradient echo sequence (field-of-view=40cm, flip angle=12˚, matrix=256x160x128, slice thick=3.0mm, TR=7.1 ms, TE=2.9ms).
Two vials with 40- and 80-mM sodium concentrations were placed near the volunteers. A linear model of the phantom sodium signal intensities was used to estimate the in-vivo tissue sodium concentrations. A TR of approximately 3-times the tissue sodium T1 relaxation times was used to avoid T1 biasing of the sodium concentration estimates.
Results
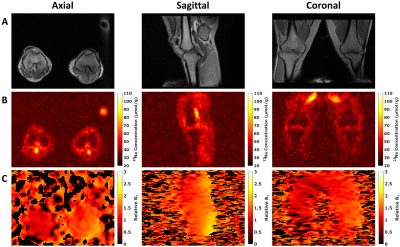
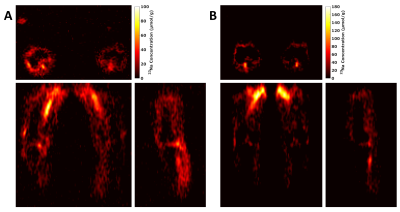
Figure 1 shows single slices of the axial, sagittal and coronal views of the 3D 1H-GRE acquisition (Figure 1, A), the estimated sodium concentration maps (Figure 1, B) and the relative B1 maps (Figure 1, C). Images are of a single volunteer (aged 34) and are of similar location.Figure 2 shows the estimated sodium concentration maps in the axial, coronal and sagittal views of two volunteers. Images in Figures 2A are of a volunteer aged 28, and images in Figure 2B of a volunteer aged 27.
Discussion
We demonstrated the ability to perform large field-of-view sodium imaging, which could enable an improved assessment of sodium related changes in cartilage, muscle, synovial fluid, and blood.The B1 maps show that the actual flip angle varied between both legs and across the field-of-view. The largest flip angle deviations were seen at the posterior areas of the legs near the coil rungs. The legs could be positioned closer to axial isocentre with additional padding to obtain more uniform B1 fields, enabling more accurate estimations of sodium concentrations.
The low spatial resolution does not allow detailed analysis of the joint. The low resolution here results in partial volume effects that confound quantitative analysis of regions of interest such as cartilage. Better resolution could be obtained with longer acquisition times or with additional local receiver coils that could be used in conjunction with the used large field-of-view transmit coil. Although the resolutions were low, the large field-of-view enabled imaging of both knees, and from hip to knee within a single acquisition.
Conclusion
This work shows the preliminary results of bilateral sodium MR imaging of the lower extremities. A single sodium acquisition could allow simultaneous biochemical assessment of different regions and tissues of the legs. The ability of sodium MRI to non-invasively quantify variations in GAG concentration could potentially assist in monitoring multiple diseases affecting the musculoskeletal system.Acknowledgements
This work was supported by GlaxoSmithKline, Cancer Research UK, Addenbrooke's Charitable Trust, and the National Institute of Health Research Cambridge Biomedical Research Centre.References
1. Shapiro EM, Borthakur A, Gougoutas A, et al. 23Na MRI Accurately Measures Fixed Charge Density in Articular Cartilage. Magn Reson Med 2002; 47: 284–291.
2. Wang C, McArdle E, Fenty M, et al. Validation of Sodium Magnetic Resonance Imaging of Intervertebral Disc. Spine (Phila Pa 1976) 2010; 35: 505–510.
3. Noebauer-Huhmann IM, Juras V, Pfirrmann CWA, et al. Sodium MR Imaging of the Lumbar Intervertebral Disk at 7 T: Correlation with T2 Mapping and Modified Pfirrmann Score at 3 T - Preliminary results. Radiology 2012; 265: 555–564.
4. Juras V, Zbýň Š, Pressl C, et al. Sodium MR Imaging of Achilles Tendinopathy at 7 T: Preliminary Results. Radiology 2012; 262: 199–205.
5. Madelin G, Babb J, Xia D, et al. Articular Cartilage: Evaluation with Fluid-suppressed 7.0-T Sodium MR Imaging in Subjects with and Subjects without Osteoarthritis. Radiology 2013; 268: 481–491.
6. Madelin G, Xia D, Brown R, et al. Longitudinal study of sodium MRI of articular cartilage in patients with knee osteoarthritis: initial experience with 16-month follow-up. Eur Radiol 2018; 28: 133–142.
7. Weber MA, Nagel AM, Wolf MB, et al.
Permanent muscular sodium overload and persistent muscle edema in Duchenne
muscular dystrophy: A possible contributor of progressive muscle degeneration. J
Neurol 2012; 259: 2385–2392.
Figures