2839
23Na MRI of human intervertebral cervical discs in Mucopolysaccharidosis type II1High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Clinical Division for Endocrinology and Metabolism, Department of Medicine III, Medical University of Vienna, Vienna, Austria, 3CD Laboratory for Clinical Molecular MR Imaging, Vienna, Austria
Synopsis
Spinal skeletal disease is one of least therapy responding symptoms in MPS patients. Cervical spine of three MPS II patients and three matched controls was measured using 23Na MRI at 7T. There was a significant difference in intervertebral discs of MPS II patient compared to controls. 23Na MRI represents a potential quantitative method for assessment of cervical disc degeneration in MPS II patients and could serve as a potential marker used in monitoring of emerging therapies.
Introduction
Mucopolysaccharidosis (MPS) is a group of lysosomal storage disorders characterized by deficient activity of enzymes that degrade glycosaminoglycans (GAGs). Although the available therapies for MPS help with most of the symptoms, it’s not the case for the spinal manifestation of the skeletal disease in these patients, since the discs and bones are not as well vascularized as other tissues1. Using sodium (23Na) MRI it is possible to quantitatively and non-invasively evaluate GAG content in cartilage2, therefore this technique could serve as a possible method in the assessment of intervertebral disc status, in the spine of MPS patients, representing how much a patient’s skeletal system is affected.The aim of this study was to find if 23Na-MRI is able to detect sodium signal difference in cervical intervertebral discs between MPS II patients their sex- and age-matched controls.Material and Methods
After giving written and oral consent, three MPS II patients and their sex and age-matched controls (27+/-11) had been measured using 7T MRI (Magnetom, Siemens Healthineers, Erlangen, Germany) with a 6-channel 23Na-tuned surface spine coil (QED, Mayfield Village, Ohio).A 3D gradient echo sequence with variable echo time train (vTE-GRE) optimized for 23Na signal was acquired with the following parameters: resolution 2.5⨯2.5⨯8.0mm3, 14 averages, TR/TE 130/0.72ms at the center of the k-space, pulse bandwidth 120Hz/Px, FA 90°, acquisition time of 21 min 3. For B1 field correction in 23Na-MRI, a homogeneous cylinder phantom (0.9% NaCl) measurements were performed.
23Na images were corrected by normalizing using images from phantom measurement. Polygonal ROIs were manually drawn around the discs and their average value was normalized to CSF acquired from an ROI placed in the fourth ventricle. Image processing and ROI positioning in the discs, was done using an in-house script written in MATLAB (Mathworks Inc, Natick, MA). Shapiro-Wilk normality test was used to define the normality of the data and then a paired t-test was used to compare patients with matched controls. Analysis of covariance was used to assess the possible covariance of age and/or disc position. Statistical analysis was performed in RStudio (RStudio, Boston, MA).
Results
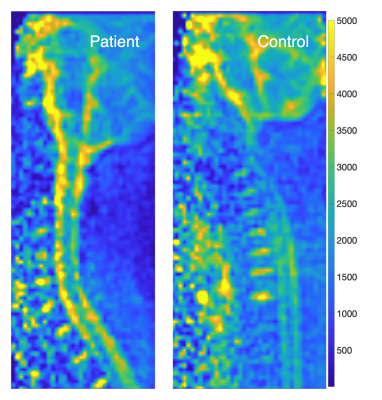
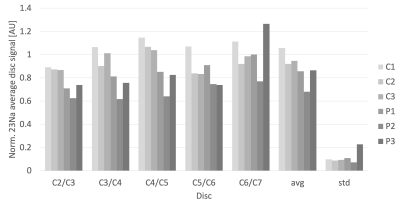
Examples of sagittal 23Na-MRI map from a MPS II patient and control (subject pair P1/C1 in Fig. 2) are displayed on the Figure 1. The average disc values calculated from ROIs drawn on 23Na-MRI maps from all the discs (C2/C3 – C5/C6) of all subjects, can be seen on the Figure 2, along with the average values and standard deviations for each subject. The C6/C7 disc data were excluded from the analysis due to possible bias caused by breathing artifacts.Analysis of covariance didn’t show a significant effect of age, or the disc placement to the acquired and normalized 23Na values from disc ROIs (p = 0.225 for disc number and 0.872 for age).The normalized disc 23Na MRI values in MPS II patients were found to be significantly lower than in their matched controls (p < 0.01).
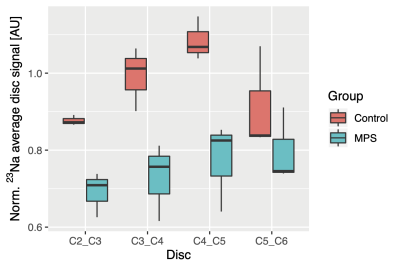
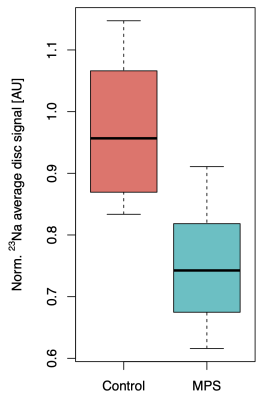
Boxplots representing data from all cervical discs in MPS II patients and their controls can be seen on Figure 3. Boxplot pooling all the discs from subjects and controls respectively, can be seen on Figure 4.
Discussion and Conclusion
It is well known that MPS II patients exhibit evidence of intervertebral disc degeneration. A decrease signal intensity was found on T2-weighted MRI images4. Accelerated intervertebral disc degeneration was found as well in animal MPS models1.Young MPS VII dogs exhibit enlarged nucleus pulposus and increased water and GAG content in anulus fibrosus, in older age they did show advanced degenerative changes in intervertebral discs, including complete disc height collapse1. Additionally, 23Na has been shown to be able to detect signs of intervertebral disc degeneration2. Our results support both previous findings. We have found significantly lower 23Na values in cervical discs of MPS II patients compared to healthy controls. This indicates that sodium MRI has a potential as a novel quantitative and non-invasive marker for disc degeneration in MPS II patients.Typical treatment options for MPS patients are bone marrow transplantation and enzyme replacement therapy. One of the challenges is the lower efficacy of these techniques in affecting the spine, where tissues are mostly avascular and need to use diffusion to deliver the treatment into the cells1. Based on our results, 23Na MRI in cervical discs might serve as a biomarker for monitoring new emerging therapies.
The major limiting aspect of our study was the 23Na surface coil design, causing signal loss from more distant tissues. This can be better observed in the Figure 1, where the noise visibly increases at a certain level away from the coil in both the patient and the control. This increase in noise will affect data especially in subjects with more curved spine or higher BMI. The solution would be to improve the coil design for future studies.
To conclude, 23Na MRI represents a potential quantitative method for assessment of cervical disc degeneration in MPS II patients and could serve as a potential marker used in monitoring of emerging therapies.
Acknowledgements
We acknowledge financial support by the OeNB (grant #13418). AS was supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 794986.References
1. Peck SH, Casal ML, Malhotra NR, Ficicioglu C, Smith LJ. Pathogenesis and treatment of spine disease in the mucopolysaccharidoses. Mol Genet Metab. 2016;118(4):232–243. doi:10.1016/j.ymgme.2016.06.0022.
2. Zbyn, S. et al. Sodium MR Imaging of Articular Cartilage Pathologies. Curr Radiol Rep, 2014. 2: p. 41.3.
3. Deligianni X, Bar P, Scheffler K, et al. High-resolution Fourier-encoded sub-millisecond echo time musculoskeletal imaging at 3 Tesla and 7 Tesla. Magn Reson Med. 2013;70(5):1434–1439. doi: 10.1002/mrm.24578.4.
4. Zafeiriou DI, Batzios SP. Brain and spinal MR imaging findings in mucopolysaccharidoses: a review. American Journal of Neuroradiology. 2013;34:5–13.
Figures