2829
A rat model of periprosthetic joint infection (PJI) using 3D printed titium hip implants: Ultrashort echo time (UTE) MRI at 7T1Ottawa Hospital Research Institute, Ottawa, ON, Canada, 2Orthopedic Surgery, The Ottawa Hospital, Ottawa, ON, Canada, 3Medical Imaging, The Ottawa Hospital, Ottawa, ON, Canada, 4Radiology, University of Ottawa, Ottawa, ON, Canada, 5Radiology, University of California at San Francisco, San Francisco, CA, United States, 6Western University, London, ON, Canada, 7Robarts Research Institute, London, ON, Canada
Synopsis
Two to fourteen percent of primary total joint replacement cases develop periprosthetic joint infection (PJI), a devastating complication. PJI animal models for hip and knee are required to evaluate prevention and treatment methods. We developed a novel rat model for PJI using a 3D printed titanium hip implant. Implant stability in the rats was monitored with 7T MRI using Ultrashort Echo Time (UTE) imaging and classical spin echo imaging. UTE demonstrated superior image quality compared to spin echo imaging. This rat model will provide a unique opportunity to validate and study strategies focusing on the prevention and treatment of PJI.
Introduction
Total joint replacement (TJR) of the hip or knee is a common orthopedic procedure that provides significant improvements in patients’ quality of life [1, 2]. Unfortunately, 2-14% of primary TJR cases develop periprosthetic joint infection (PJI), a devastating complication that requires morbid revision surgery [3, 4]. Staphylococcus species are the most commonly isolated bacteria in PJI. Biofilm formation on implants is a major contributor to treatment failure. Biofilms protect bacterial cells from antibiotics, antiseptics, host immune system and mechanical treatment. PJI animal models for hip and knee are required to evaluate conventional PJI prevention and treatment methods as well as novel therapeutic strategies. The aim of this study was to develop a clinically representative peri prosthetic joint infection in vivo model of partial hip replacement in rats using 3D-printed clinical grade titanium implants. The integration of bone and titanium was monitored over time using Ultrashort Echo Time (UTE) MRI at 7T.Methods
We performed partial hip replacement surgery on Sprague-Dawley rats using 3D -printed rat-specific medical-grade titanium Ti-6Al-4V implants (Fig 1). PJI was established by injecting in the intra-articular space 107-108 CFU bioluminescent Staphyloccus aureus Xen36. This strain allowed for real-time detection of infection using an IVIS Spectrum optical imaging system. Three different cohorts were investigated: A) naive control [no hip surgery, no infection]. ; B) surgical control [hip surgery but no infection] and C) infection [hip surgery + infection]. To confirm the location and stability of the implant, axial MRI images were acquired with a 7T GE/Agilent Discovery MR901. A 15 cm inner diameter birdcage coil was used for signal transmission and a 3 cm surface coil for signal reception. In an attempt to reduce metal artifacts, a classical 2D spin echo sequence was used with TE=8.2 ms, TR=1000 ms, bandwidth=125 kHz, FOV=7 cm, thick=1 mm, spacing=0.1 mm, matrix=512x512, scan time=9 min. Additionally, a 3D UTE sequence was run with TE=0.08 ms, TR=12 ms, flip=15 deg, FOV=45×36×39 mm3, receiver bandwidth = 250 kHz, spatial resolution = 0.2×0.2×0.75 mm3, scan time = 10 min. MRI images were reviewed to evaluate the location of the implant within the tissue over time. Real-time monitoring of infection was performed using the IVIS at different time intervals. Animals were sacrificed at 3 or 12 weeks post infection. PJI was evaluated and quantified by histologic analysis of the peri-implant tissue (bone and capsule), as well as by homogenizing peri-implant tissues and plating on agar to quantify viable bacteria.Results
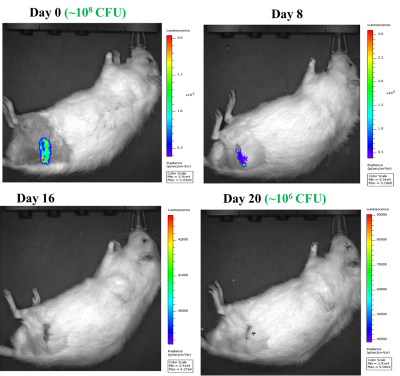
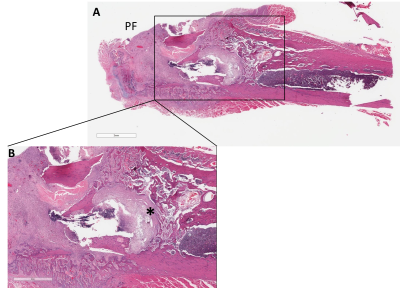
We were successful in performing partial hip replacement surgeries in Sprague-Dawley rats using 3D printed titanium implants. MRI confirmed implant stability within the femoral canal and native acetabulum (2nd and 3rd Figures). Although artifacts from the implant are visible on the spin-echo and UTE images, UTE images demonstrated superior image quality for evaluating the location of the implant compared to spin echo. The presence of S. aureus infection was confirmed for at least 3 weeks using IVIS imaging (4th Figure). This was also confirmed using gross dissection post-mortem and viable bacterial count (data not shown). Histology sections confirmed the development of inflammatory reaction and osteomyelitis in the femur at 3 weeks post-surgery+ bacterial inoculation (last Figure).Conclusion
We developed a novel rat model for peri-prosthetic joint infection (PJI) using a 3D printed titanium hip implant. Implant stability in the rats was monitored with 7T MRI using Ultrashort Echo Time (UTE) imaging and classical spin echo imaging. UTE demonstrated superior image quality compared to spin echo imaging. This rat model will provide a unique opportunity to validate and study strategies focusing on the prevention and treatment of PJI.Acknowledgements
No acknowledgement found.References
1. Norman-Taylor FH, Palmer CR, Villar RN. Quality-of-life improvement compared after hip and knee replacement. J Bone Joint Surg Br Vol. 1996;78(1):74.
2. Martinez-Cano JP, Herrera-Escobar JP, Arango Gutierrez AS, Sanchez Vergel A, Martinez-Rondanelli A. Prospective quality of life assessment after hip and knee arthroplasty: short- and mid-term follow-up results. Arthroplast Today. 2017;3(2):125.
3. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infect ion. Lancet (Lond Engl). 2016;387(10016):386.
4. Berend KR, Lombardi AV Jr,Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471(2):510.
Figures