2809
Ultrashort echo time adiabatic T1ρ (UTE-Ad-T1ρ) can predict the mechanical property differences in human anterior cruciate ligament (ACL)
Saeed Jerban1, Takehito Hananouchi2, Yajun Ma1, Erik Dorthe3, behnam namiranian1, Jonathan Wong4, Mei Wu1, Darryl D'lima3, Eric Y Chang4, and Jiang Du1
1Radiology, University of California, San Diego, San Diego, CA, United States, 2Department of Mechanical Engineering, Osaka Sangyo University, Daito, Osaka, Japan, 3Shiley Center for Orthopedic Research and Education at Scripps Clinic, La Jolla, CA, United States, 4Research Service, VA San Diego Healthcare System, San Diego, CA, United States
1Radiology, University of California, San Diego, San Diego, CA, United States, 2Department of Mechanical Engineering, Osaka Sangyo University, Daito, Osaka, Japan, 3Shiley Center for Orthopedic Research and Education at Scripps Clinic, La Jolla, CA, United States, 4Research Service, VA San Diego Healthcare System, San Diego, CA, United States
Synopsis
Clinical MRI techniques typically show the anterior cruciate ligament (ACL) as low in signal. Commonly used quantitative MRI techniques are sensitive to tissue orientation and magic angle effect. A recently developed ultrashort echo time (UTE)-based adiabatic T1ρ technique (UTE-Ad-T1ρ) can detect high signal in the ACL and may be less sensitive to magic angle effect. We have investigated for the first time the correlation between UTE-Ad-T1ρ and ACL mechanical properties. T1ρ showed significant strong correlation with average elastic modulus of ACL specimens. The UTE-Ad-T1ρ technique is highlighted as a potential tool to noninvasively assess the ACL mechanical properties.
Introduction
The anterior cruciate ligament (ACL) is comprised of a predominantly parallel arrangement of collagen fibers. Organized collagen fibers can change significantly with ACL injury and overuse. Age-related overuse injuries can also result in collagen fiber disorganization, disruption, and neovascularization which can impair the normal joint’s performance (1). These changes often can be detected semi-qualitatively using clinical MRI, based on the local signal changes in the tissue (1). Quantitative MRI-based assessment of ACL has been challenging, firstly because clinical MRI is not capable of imaging normal ACL with high signal due to short T2 (1,2), and secondly because the common quantitative MRI measures like T2 and T1ρ are orientation sensitive and affected by magic angle phenomena (3). Ultrashort echo time MRI (UTE-MRI) can be used to image the ACL (at TE<50 μs) for quantitative assessment (2,4,5). We have developed a new T1ρ sequence using an adiabatic inversion recovery spin-lock pulse cluster followed by Cones data acquisition (3D UTE-Ad-T1ρ) (6). Adiabatic pulses provide a robust spin-lock and can achieve a uniform inversion, which is widely used to create image contrast. Recent studies show that adiabatic spin-lock has the potential for orientation-insensitive T1ρ measurement (7–9). This study aims to investigate the correlations between the UTE-Ad-T1ρ biomarker (5) and mechanical properties in human ACL specimens. This study highlights the potential applications of UTE-Ad-T1ρ technique to noninvasively assess the mechanical properties of human ACL.Methods
ACL specimens were harvested from thirteen fresh-frozen human cadaveric knee joints (50±21 years old, 2 female and 11 male donors). ACL specimens were soaked in phosphate-buffered saline (PBS) for 2 hours before MRI scans to ensure rehydration after potential drying throughout the sample preparation. Specimens were scanned on a 3T MRI (MR750, GE Healthcare Technologies, WI) scanner using a Mayo Clinic BC-10 T/R birdcage coil. ACL specimens were placed perpendicular to B0. A set of 3D UTE-Ad-T1ρ sequences with seven different spin-locking times (TSLs) (TSL= 0, 2, 4, 6, 8, 12 and 16 ms) were performed. Other imaging parameters included: rectangular RF excitation pulse with a duration of 26 µs, repetition time (TR)=500 ms, echo time (TE)=0.032 ms, flip angle (FA)=10˚, field of view (FOV) = 70 mm × 70 mm, matrix=300×300, slice thickness=1 mm, number of slices=46, receiver bandwidth=±62.5 kHz. T1ρ value was calculated for each specimen using a single-component exponential fitting model (MRI signal decay over increasing TSL). After MRI scans, specimens were washed with PBS before running mechanical tests. Specimens were trimmed into 7×5 mm2 approximate cross-sections (one specimen per donor) and fixed into the grippers (distance between grippers was 15mm) of an in-house developed benchtop uniaxial loading device. After initial alignment and preloading, a 1-mm tensile displacement was applied at 2 mm/min rate and the maximum applied force was recorded. Average maximum mechanical stress was calculated by the maximum load divided by ACL cross-section. The average maximum strain was 0.067 (i.e., 1 mm / 15 mm). The average tensile elastic modulus was calculated by average maximum stress divided by average maximum strain. Pearson’s correlation coefficient between UTE-Ad-T1ρ and average tensile elastic modulus was calculated. Data analysis was performed in MATLAB (2017, The Mathworks Inc., MA).Results
Figure 1a shows the UTE-Ad-T1ρ MRI image of the thirteen ACL specimens in axial plane. Figures 2a and 2b show the T1ρ fitting results for two representative ACL specimens from a 27-year-old-male and a 44-year-old-female donor, respectively. Figures 3 demonstrates the scatter plot and linear regression analysis of the average elastic modulus on UTE-Ad-T1ρ. The correlation between T1ρ and mechanical measures was significant and strong (R=-0.81, p<0.01).Discussion
Our developed UTE-Ad-T1ρ MRI technique predicted the mechanical properties of the ACL with a significant strong correlation. This study highlighted the UTE-Ad-T1ρ technique as a useful quantitative method to assess ACL mechanical properties. Future studies will be required to investigate the correlations between our MRI biomarkers as measured in vivo compared with arthroscopic measures.Conclusion
The presented UTE technique can predict mechanical properties of human ACL. Such an MRI technique with low magic angle sensitivity may help diagnose ACL injuries and monitor ACL graft maturation.Acknowledgements
The authors acknowledge grant support from NIH (R21AR073496, R01AR075825, 2R01AR062581, 1R01 AR068987), VA Clinical Science and Rehabilitation R&D Awards (I01CX001388 and I01RX002604), and JSPS KAKENHI Grant Number JP18KK0104.References
1. Fleming BC, Biercevicz AM, Murray MM, Li W, Wang VM. Emerging Techniques for Tendon and Ligament MRI. Magn. Reson. Imaging Tissue Eng. 2017:209–236. doi: 10.1002/9781119193272.ch10. 2. Chang EY, Du J, Chung CB. UTE imaging in the musculoskeletal system. J. Magn. Reson. Imaging [Internet] 2015;41:870–883. doi: 10.1002/jmri.24713. 3. Shao H, Pauli C, Li S, Ma Y, Tadros AS, Kavanaugh A, Chang EY, Tang G, Du J. Magic angle effect plays a major role in both T1ρ and T2 relaxation in articular cartilage. Osteoarthr. Cartil. [Internet] 2017;25:2022–2030. doi: 10.1016/j.joca.2017.01.013. 4. Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed. [Internet] 2013;26:489–506. doi: 10.1002/nbm.2906. 5. Ma Y, Carl M, Searleman A, Lu X, Chang EY, Du J. 3D adiabatic T1ρprepared ultrashort echo time cones sequence for whole knee imaging. Magn. Reson. Med. 2018;80:1429–1439. doi: 10.1002/mrm.27131. 6. Ma Y, Carl M, Shao H, Tadros AS, Chang EY, Du J. Three-dimensional ultrashort echo time cones T 1ρ (3D UTE-cones-T 1ρ ) imaging. NMR Biomed. [Internet] 2017;30:e3709. doi: 10.1002/nbm.3709. 7. Casula V, Autio J, Nissi MJ, Auerbach EJ, Ellermann J, Lammentausta E, Nieminen MT. Validation and optimization of adiabatic T1ρ and T2ρ for quantitative imaging of articular cartilage at 3 T. Magn. Reson. Med. 2017;77:1265–1275. doi: 10.1002/mrm.26183. 8. Rautiainen J, Nissi MJ, Liimatainen T, Herzog W, Korhonen RK, Nieminen MT. Adiabatic rotating frame relaxation of MRI reveals early cartilage degeneration in a rabbit model of anterior cruciate ligament transection. Osteoarthr. Cartil. [Internet] 2014;22:1444–1452. doi: 10.1016/j.joca.2014.04.023. 9. Liimatainen T, Sorce DJ, O’Connell R, Garwood M, Michaeli S. MRI contrast from relaxation along a fictitious field (RAFF). Magn. Reson. Med. [Internet] 2010;64:983–994. doi: 10.1002/mrm.22372.Figures
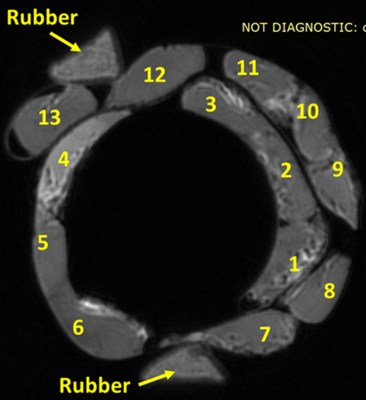
Figure
1: UTE-MRI image of thirteen anterior cruciate ligament (ACL) specimens from
different human donors (50±21 years old). (a) UTE-MRI (TE=0.032ms) image of a
set of twenty cortical bone strips harvested from different donors with 4×2 mm2
cross-sections (250 μm in plane pixel size) on average soaked in fomblin, which
has no signal in MRI. UTE scans of the specimens was performed in the presence
of a rubber phantom with known proton density (30 mol/L). (b) μCT image of the
same set of bone specimens at 9 μm voxel size.
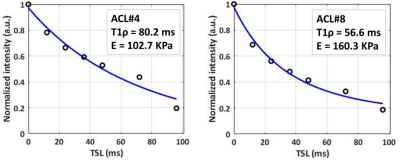
Figure
2: Adiabatic UTE-T1ρ fitting results for two representative ACL specimens.
(A) ACL#4: 27-year-old-male and (B) ACL#8: 44-year-old-female with 102.7 and
160.3 KPa elastic modulus, respectively.
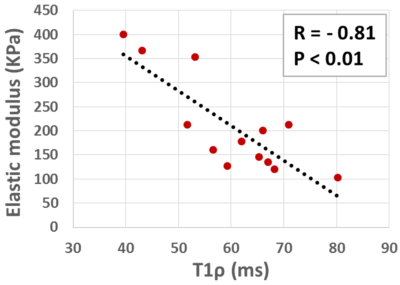
Figure
3: Scatter plot and linear regression analysis with significant correlations
(p<0.01) of average elastic modulus on T1ρ.