2802
Magic Angle Directional Imaging (MADI) visualises changes to collagen fibre orientation in patellar tendons after freezing
Karyn Elizabeth Chappell1, Mihailo Ristic2, Donald McRobbie3, Wladyslaw Gedroyc4, Djordje Brujic2, and Catherine Van Der Straeten5
1Medicine, Orthopaedic Surgery, Stanford University, Redwood City, CA, United States, 2Mechanical Engineering, Imperial College London, London, United Kingdom, 3University of Adelaide, Adelaide, Australia, 4Imperial College London, London, United Kingdom, 5Imperial College London/Ghent University, Ghent, Belgium
1Medicine, Orthopaedic Surgery, Stanford University, Redwood City, CA, United States, 2Mechanical Engineering, Imperial College London, London, United Kingdom, 3University of Adelaide, Adelaide, Australia, 4Imperial College London, London, United Kingdom, 5Imperial College London/Ghent University, Ghent, Belgium
Synopsis
Does freeze/thaw of cadaveric specimens’ damage collagen fibre orientation? Magic angle directional imaging (MADI) in caprine knees assessed the underlying tissue structure before and after freezing for three and six weeks. Tendon thickness reduced after 6 weeks of freezing. Segmented collagen containing voxels decreased by half after freezing. Voids appeared in the internal tendon structure suggestive of ice crystal formation that disrupted collagen fibre orientation. The severity of the structural changes increased the longer the tissue was frozen. Interpreting results with frozen/thawed cadaveric specimens needs care as freezing damages collagen fibre structure that may impact on biomechanical and other properties.
Introduction
Cadaveric tissue preserved by chemical fixation or freezing is widely used for developing musculoskeletal (MSK) research. Limbs are frozen then thawed before scanning during MR imaging experiments. Van der Made et al. found that freeze thawing does not affect the MR image quality unless repeated too often1. However, in rabbit patellar tendons biomechanical and structural changes to tissues after freezing at -80˚C worsened the longer the tissue was frozen2. When Chang et al. studied tendons they found no significant changes to T2 or T2* after five freeze/thaw cycles3 but Powdner et al. measured a 12% reduction to T2* after multiple cycles4. In developing the MADI technique we investigated if freeze/thawed tissue could be utilised for a repeatability study or does the freeze/thaw process alter the collagen structure of tendons?Methods
The effect of freezing on collagen fibre structure was explored using two fresh caprine knees that were scanned then frozen at -20˚C for a period of three or six weeks before being thawed and re-scanned. The two time points were chosen to reflect the changes in collagen fibre bundles noted by Tsuchida et al.2. The caprine knees were scanned in nine orientations using the MADI technique5. Fibre orientation maps (FOM) of the segmented patellar tendon before and after freezing were computed using Matlab 2015b. 1mm3 voxel glyphs showing net collagen fibre orientation in the patellar tendons were computed using ParaView 5.1.2. Comparison of FOM and glyphs before and after freezing at the two time points are visualised.Results
No changes to MR image quality were noted between the two caprine knees before and after freezing. The thickness of the patellar tendon remained unchanged after three weeks of freezing. However after six weeks of freezing the patellar tendon thinned and altered signal intensity occurred within the trabecular bone in the tibial growth plate (fig1). On the FOM voxel voids are seen in the patellar tendon three weeks after freezing but not in the fresh specimen (fig2A). At six weeks after freezing the voxel voids on the FOM are more prevalent with larger spaces opening up within the tendon (fig2B). Voxel voids indicate areas where the magic angle effect has disappeared because the voxels are no longer exhibiting a variation in signal intensity with the MADI technique. Segmented voxels decreased from fresh to frozen tissue. Fresh samples had 1452 and 2053 voxels respectively which decreased by 43% (824 voxel) at three weeks and 56% (909 voxels) at six weeks. ParaView glyph visualisation of the tendons before and after freezing demonstrated a change in thickness and damage to the collagen fibre structure (coronal and axial images) which is more pronounced at six weeks (fig3).Discussion
There was little variation in MR image quality of the 3D T1-W images between the freshly scanned and freeze/thawed caprine knees. This finding is in agreement with a number of other researchers6, 7, 8, 9. Powdner et al. demonstrated that multiple freeze thaw cycles reduced T2* indicative of collagen structure alteration4. This was why a single freeze thaw cycle was used for this work. However there was a notable change in thickness of the patellar tendon after six weeks of freezing that may reflect changes to the collagen structure.Voxel voids on the FOM and ParaView glyphs after freezing are suggestive of ice crystal formation that caused interfibrillar gaps to form in the tendon2, 10, 11. Giannini et al. noted a decrease in collagen fibrils after freezing as ice crystals caused swelling, splitting and fragmentation of the collagen bundles histologically11. Tsuchida et al. found freezing significantly altered the microstructure of normal patellar tendon after 3 weeks and the ultrastructure after 6 weeks2. Our findings are in line with Giannini et al. and Tschida et al.11,2. An alteration of the caprine patellar tendon structure was detected after freezing. Unfortunately without a histological reference which was not possible in this study a definitive conclusion was not possible. It would also be difficult to say with certainty due to the one millimetre resolution whether the ultrastructure of collagen had altered because it was not measured. The longer the tendon was frozen the less voxels were segmented (decreased by 56%) and the greater the number of voxels voids within the tendon, which is suggestive of damage to collagen fibre structure.
Conclusion
Frozen/thawed cadaveric tissue is commonly used in MSK MRI research development. Careful interpretation of results concerning collagen fibre structure after freezing is necessary. Ice crystals may alter the hydrogen bonding on the surface of the protein molecular structure causing damage to the collagen fibre bundles. The severity of the structural changes appears to increase with the length of time the tissue is frozen before being thawed and scanned. Increasing tissue longevity by freezing then thawing in repeatability experiments is not advisable due to damage of the collagen fibre structure after freezing for three and six weeks. Further work may be useful to assess shorter periods of freezing such as two weeks or less. A faster freeze method with colder storage temperature (i.e. -80˚C) could also be assessed to determine if that reduced the ice crystal formation and damage.Acknowledgements
This work was supported by the National Institute for Health Research (NIHR) Invention for Innovation (i4i) under Grant II-LA-1111-20005. We are grateful to Charing Cross Hospital MRI department and Imaging Committee for the kind use of the Siemens 3T Verio.References
- Van Der Made, A. D., Maas, M., Beenen, L. F., Oostra, R. J. & Kerkhoffs, G. M. (2013) Post-mortem imaging exposed: an aid in MR imaging of musculoskeletal structures. Skeletal Radiol 42, 467-472.
- Tscuchida, T., Yasuda, K., Kaneda, K., Hayashi, K., Yamamoto, N., Miyakawa, K. & Tanaka, K. (1997) Effects of in situ freezing and stress‐shielding on the ultrastructure of rabbit patellar tendons. J Orthop Res 15, 904-910.
- Chang, E. Y., Bae, W.C., Stanum, S., Du, J. & Chung, C. B. (2014) Effects of repetitive freeze-thawing cycles on T2 and T2* of the Achilles tendon. Euro J Radiol 83, 349-353.
- Pownder, S. L., Shah, P. H., Potter, H. G. & Koff, M. F.
(2015) The effect of freeze-thawing on magnetic resonance imaging T2* of
freshly harvested bovine patellar tendon. Quant Imaging Med Surg 5, 368-373.
- Chappell, K.E., Brujic, D., Straeten, C.V.D., Meeson, R., Gedroyc, W., McRobbie, D., Ristic, M. (2019) Detection of maturity and ligament injury using Magic Angle Directional Imaging (MADI). Magnet Reson Med, 82, 1041-1054.
- Hodler, J. H., P. Trudell, D. & Resnick, D. (1992) The cruciate ligaments of the knee correlation between MR appearance gross histologic findings in cadaveric specimens. Am J Roentgenol 159, 357-360.
- Hodler, J. T., D., Sik Kang, H., Kjellin, I. & Resnick, D. (1992) Inexpensive Technique for Performing Magnetic Resonance -Pathologic Correlation in Cadavers. Invest Radiol 27, 323-325.
- Widmer, W. R.., Fessler, J.F. & Ivancevich, S. (1999) A Technique for MRI of Equine Cadaver Specimens. Veterinary Radiol Ultrasound 40, 10-14.
- Bolen, G.E., Haye, D., Dondelinger, R. F., Massart, L. & Busoni, V. (2011) Impact of successive freezing-thawing cycles on 3-T magnetic resonance images of the digits of isolated equine limbs. Am J Vet Res 72, 780-790.
- Park, H. J., Urabe, K., Naruse, K., Onuma, K., Nemoto, N. & Itoman, M. (2009) The effect of cryopreservation or heating on the mechanical properties and histomorphology of rat bone-patellar tendon-bone. Cell Tissue Bank 10, 11-18.
- Giannini, S., Buda, R., Di Caprio, F., Agati, P., Bigi, A., De Pasquale, V. & Ruggeri, A. (2008) Effects of freezing on the biomechanical and structural properties of human posterior tibial tendons. Int Orthop 32, 145-151.
Figures
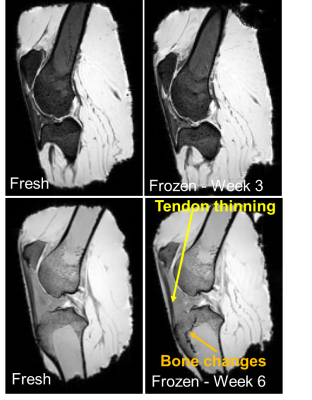
Figure 1: MR images of the
caprine knees before freezing and then after freezing at three weeks and six weeks.
Image quality and
contrast remains similar after freezing. Patellar tendon has a similar appearance
at three weeks. Muscle, bone and fat demonstrate no change in contrast after
freezing. The main differences are the thickness of the patellar tendon after
six weeks appears to be reduced and the bone marrow around the fused epiphyseal plate
has a series of hypointense black holes.
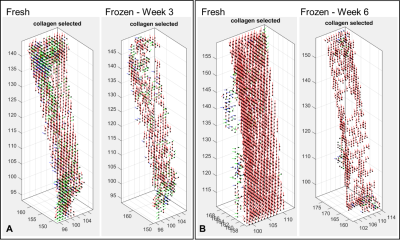
Figure 2: Fibre orientation
maps (FOM) of segmented collagen voxels for patellar tendons before and after
freezing for three weeks [A] and six weeks [B].
Patellar tendons in the fresh samples demonstrate
highly aligned collagen voxels in an up-down orientation. After freezing the
patellar tendon has voxel voids within it indicating a loss of the magic angle
effect in the collagen voxels inside the patellar tendon. Prevalence of the
voxel void increases the longer the sample is frozen.
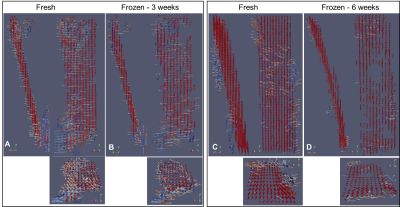
Figure 3 ParaView glyph
images in sagittal, coronal and axial planes for the fresh and frozen patellar
tendons.
[A/B] three weeks
after freezing there is little change in patellar tendon thickness but a few
voxel voids are noted on the coronal image. [C/D] six weeks after freezing
there is a marked thinning of the patellar tendon on the sagittal plane and a
large number of voxel voids on the coronal plane. The longer the tendon is
frozen the more damage occurs to the internal structure.