2774
Predicting cortical bone microstructural properties by water proton density obtained from ultrashort echo time (UTE) MRI
Saeed Jerban1, Yajun Ma1, Hyungseok Jang1, behnam namiranian1, Nicole Le2, Hoda Shirazian1, Mark Murphy3, Jiang Du1, and Eric Y Chang2
1Radiology, University of California, San Diego, San Diego, CA, United States, 2Radiology Service, VA San Diego Healthcare System, San Diego, CA, United States, 3Orthopaedic Service, VA San Diego Healthcare System, San Diego, CA, United States
1Radiology, University of California, San Diego, San Diego, CA, United States, 2Radiology Service, VA San Diego Healthcare System, San Diego, CA, United States, 3Orthopaedic Service, VA San Diego Healthcare System, San Diego, CA, United States
Synopsis
Cortical bone porous microstructure can be potentially predicted by the total water density in bone. Comparing bone signal from a relatively fast ultrashort echo time MRI (UTE-MRI) scan against the signal of a known external reference (rubber eraser) enabled us to measure total water proton density (TWPD) in 135 cortical bone specimens from 37 donors. We observed significant correlations between bone TWPD and micro computed tomography (μCT) measures (porosity, pore size, and bone mineral density, (BMD)). This relatively fast MRI technique may aid diagnosing and monitoring bone diseases.
Introduction
MRI-based cortical bone evaluation is attractive since MRI is tomographic and avoids the potential harm associated with x-ray-based techniques. MRI-based bone evaluation may also provide excellent assessment of the surrounding soft tissue, a benefit which is not available in x-ray-based techniques. TWPD in cortical bone can be measured by comparing bone signal in ultrashort echo time MRI (UTE-MRI) against the signal of a known external reference (1,2). The external reference can be any material with a known apparent proton density and a range of MRI properties similar to bone, such as the mixture of distilled water and heavy water (3–6) and the rubber phantom. Gradual increases in the porosity of human bone due to aging or osteoporosis (OP) is hypothesized to result in an increased pore water proton density (PWPD). If we assume that independent changes in the bone’s organic matrix during aging or osteoporosis development are limited, alterations in PWPD should be seen in TWPD. Therefore, investigating only TWPD in cortical bone may be sufficient for clinically relevant bone evaluation which merely needs a single, relatively fast MRI scan. The purpose of this study was to investigate the correlations between bone microstructural properties and TWPD in a large number of human bone specimens. This study complements our earlier feasibility study performed on eight tibial bone specimens (3). Herein we highlight the potential applications of a relatively fast UTE-MRI scan to quantitatively assess human cortical bone.Methods
A total of 135 cortical bone specimens were harvested from human tibial and femoral midshafts of 37 donors (61±24 years old). Samples were scanned using 3D-UTE-Cones sequences on a clinical 3T MRI (GE Healthcare, WI, USA). Specimens were scanned together with a known rubber phantom (33 mol/L H1, T2≈1.3 ms, T1≈280 ms). Two UTE-MRI sequences were performed. First, to measure TWPD, a single UTE sequence was performed with the following acquisition parameters: rectangular RF excitation pulse with a duration of 26 µs, repetition time (TR)=100 ms, echo time (TE)=0.032 ms, flip angle (FA)=10˚, field of view (FOV)= 40mm, matrix size=160×160, in-plane pixel size=0.25mm, slice thickness=2 mm, receiver bandwidth=±62.5 kHz. Second, a 30-mL syringe filled with pure water was imaged using the UTE-MRI protocol to generate the coil sensitivity map (η) over the selected FOV. The total MRI scan time was approximately seven minutes. Since T2*bone and T2*Rub are much higher than the ultrashort TE and the rectangular excitation pulse duration, the T2* and T1 effects can be neglected; thus, TWPD can be estimated by comparing the UTE signals of bone and external reference using Eq.1. Specimens were later scanned on a μCT scanner (Skyscan 1076, Belgium) at 9 μm isometric voxel size. Other scanning parameters were as follows: 0.05-mm aluminum and 0.038-mm copper filters, 100 kV, 100 mA, 0.3˚ rotation step, and 5 frame-averaging. Average bone porosity, pore size, and BMD were measured from μCT images. Pearson’s correlation coefficients between TWPD and μCT-based measures were calculated.Results
Figure 1a shows the UTE-MRI image of a set of bone specimens in the 30-ml syringe scanned in axial plane. The rubber phantom with the known proton density was placed in the syringe for TWPD measurement which is indicated with a yellow arrow. Figure 1b shows the µCT image of the same set of samples at 9 μm isometric voxel size. Figures 2a, 2b, and 2c demonstrate the scatter plots and linear regressions of TWPD on µCT-based average bone porosity, pore size, and average BMD, respectively. TWPD demonstrated significant moderate correlation with both average bone porosity (R=0.66, p<0.01) and pore size (R=0.58, p<0.01). TWPD demonstrated significant strong with BMD (R=0.71, p<0.01).Discussion
The presented 3D-UTE-Cones imaging technique allows assessment of TWPD in human cortical bone. This quick UTE-MRI-based technique was capable of predicting bone microstructure differences with significant correlations as examined in 135 bone specimens.Conclusion
UTE-MRI-based measurement of TWPD can potentially be used to assess cortical bone microstructure with a relatively fast MRI scan time.Acknowledgements
The authors acknowledge grant support from NIH (R21AR073496, R01AR075825, 2R01AR062581, 1R01 AR068987) and VA Clinical Science and Rehabilitation R&D Awards (I01CX001388 and I01RX002604).References
1. Du J, Chiang AJT, Chung CB, Statum S, Znamirowski R, Takahashi A, Bydder GM. Orientational analysis of the Achilles tendon and enthesis using an ultrashort echo time spectroscopic imaging sequence. Magn. Reson. Imaging [Internet] 2010;28:178–184. doi: 10.1016/j.mri.2009.06.002. 2. Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed. [Internet] 2013;26:489–506. doi: 10.1002/nbm.2906. 3. Jerban S, Ma Y, Li L, Jang H, Wan L, Guo T, Searleman A, Chang EY, Du I, Du J. Volumetric Mapping of Bound and Pore Water as well as Collagen Protons in Cortical Bone Using 3D Ultrashort Echo Time Cones MR Imaging Techniques. Bone [Internet] 2019;127:120–128. doi: 10.1016/j.bone.2019.05.038. 4. Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J. Magn. Reson. [Internet] 2010;207:304–311. doi: 10.1016/j.jmr.2010.09.013. 5. Manhard MK, Horch RA, Gochberg DF, Nyman JS, Does MD. In Vivo Quantitative MR Imaging of Bound and Pore Water in cortical bone. Radiology 2015;277:221–230. 6. Zhao X, Song HK, Seifert AC, Li C, Wehrli FW. Feasibility of assessing bone matrix and mineral properties in vivo by combined solidstate 1H and 31P MRI. PLoS One 2017;12:1–16. doi: 10.1371/journal.pone.0173995.Figures

Eq1: Calculating TWPD
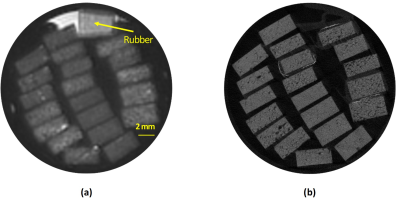
Figure
1: UTE-MRI image and μCT image of a set of twenty cortical bone strips
harvested from different donors possessing different levels of porosities. (a)
UTE-MRI (TE=0.032ms) image of a set of twenty cortical bone strips harvested
from different donors with 4×2 mm2 cross-sections (250 μm in plane pixel size)
on average soaked in fomblin, which has no signal in MRI. UTE scans of the
specimens was performed in the presence of a rubber phantom with known proton
density (30 mol/L). (b) μCT image of the same set of bone specimens at 9 μm
voxel size.
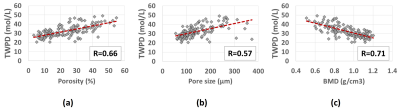
Figure
2: Scatter plots and linear regression analyses with significant correlations
(p<0.01) of total water proton density (TWPD) on µCT-based microstructural
properties. TWPD versus (a) porosity, (b) average pores size, and (c) bone
mineral density (BMD).