2758
Evaluation of bone marrow proton density fat fraction in β-thalassaemia patients and healthy subjects using 1H-MR spectroscopy.
Umi Nabilah Ismail1, Che Ahmad Azlan1, Shasha Khairullah2, Kuan Jin Lee3, Chai Hong Yeong4, Nur Farhayu Omar5, Mohammad Nazri M Shah1, Raja Rizal Azman Raja Aman1, Nicholas Jackson6, and Kwan Hoong Ng1
1Biomedical Imaging Department, University of Malaya, Kuala Lumpur, Malaysia, 2Department of Medicine, University of Malaya, Kuala Lumpur, Malaysia, 3Singapore Bioimaging Consortium, A*STAR Research, Singapore, Singapore, 4School of Medicine, Faculty of Health and Medical Sciences, Taylor's University Lakeside Campus, Subang Jaya, Malaysia, 5Department of Imaging, Universiti Putra Malaysia, Serdang, Malaysia, 6Department of Pathology, University of Malaya, Kuala Lumpur, Malaysia
1Biomedical Imaging Department, University of Malaya, Kuala Lumpur, Malaysia, 2Department of Medicine, University of Malaya, Kuala Lumpur, Malaysia, 3Singapore Bioimaging Consortium, A*STAR Research, Singapore, Singapore, 4School of Medicine, Faculty of Health and Medical Sciences, Taylor's University Lakeside Campus, Subang Jaya, Malaysia, 5Department of Imaging, Universiti Putra Malaysia, Serdang, Malaysia, 6Department of Pathology, University of Malaya, Kuala Lumpur, Malaysia
Synopsis
Fat in bone marrow is stated to be regulated by the body haematopoietic needs. We explored this by measuring bone marrow fat fraction(FF) of healthy subjects and β-thalassaemia patients who required treatment to suppress their body haematopoietic needs. The results obtained from 1H-MR Spectroscopy suggested that information about marrow adipocytes may be useful in evaluating patients' treatment response.
Introduction
β-thalassaemia is a genetic disease that is characterized by an absence or reduction of β-globin chain in haemoglobin which then resulted in anaemia1. To compensate for the anaemic condition, the body forces more red blood cells (RBCs) to be produced by the bone marrow, which cause the erythroid marrow expansion to occur. Blood transfusion is the main treatment given to patients.Marrow adipocytes, one of the main components of bone marrow, are stated to be regulated by the prevailing requirement for haematopoiesis2. The healthy ageing process has been associated with an increase in marrow adipocytes and decrease in haematopoietic needs.
However, in pathologic conditions that can cause an increase in haematopoietic needs (e.g. anaemia), the fat in bone marrow will decrease. As blood transfusion given to the patients is aimed to reduce body's haematopoietic needs, it is expected that the fat in bone marrow can be an indication for the response of the patients to the treatment given. Hence, this study aims to investigate the difference of fat fraction (FF) in proximal femur of healthy subjects and β-thalassaemia patients.
Methodology
1H MRS Spectra of four healthy subjects and five β-thalassaemia patients who needed to receive monthly blood transfusion were obtained using STEAM sequence (TR:3000ms TE: 20,25,30,35 and 40ms and TM: 10ms)3. The spectra were obtained using an 8-channel phased-array coil with a 3T Siemens MAGNETOM Prisma system. ROIs with 10x10x10 mm were drawn on the femoral head, femoral neck, diaphysis and greater trochanter for each subject (Fig 1). The spectra were analysed using AMARES algorithm4 included in the jMRUI software package5. A custom-written MATLAB code was used to assume mono-exponential decay as to obtain T2-corrected fat and water peaks and the mean FFs for each ROI were then computed.Results and Discussion
Fig. 2 shows an example of the single-voxel STEAM MR Spectra obtained from the diaphysis of healthy subjects and patients. Fig. 2b is a representative spectrum that occurs due to iron overload in thalassaemia patients as a result of the blood transfusion they received while Fig. 2c shows an almost non-existence fat in the diaphysis region of one of the patients. The difference of FF between healthy group and β-thalassaemia patients can be observed in Fig. 3 with the FF for patients show a large variation as compared to healthy subjects.As fat in bone marrow was expected to be regulated by body haematopoietic needs, the treatment given to the patients should allow the FF of patients to be almost similar to healthy subjects. However, it can be observed that patients (mean FF: 30.5±32.3%) exhibit a low FF value compared to healthy subjects (mean FF: 92.2±5.7%) and the value of FF in patients are highly variable (range FF: 0.8 – 85.4%).
Furthermore, the extremely low value of FFs in some subjects need to be highlighted as the absence of fat in the bone marrow has been cited to be the cause for extramedullary haematopoiesis (EMH)6, a known extreme stress response to hypoxia in β-thalassaemia. Based on this, it is assumed that the treatment did not completely alleviate the anaemic condition in some patients.
The present analysis still has some limitations as the relationship between marrow adipocytes and haematopoiesis has still not been well-established. Moreover, there may be other factors that influence marrow adipogenesis in β-thalassaemia patients. Other than that, using MRI multi-point Dixon technique may allow a higher advantage as the spatial variation of FF in the femur can be obtained.
Conclusion
1H MR spectroscopy enables the bone marrow composition in the proximal femur of healthy and β-thalassaemia groups to be studied. β-thalassaemia patients show a lower value and wide range of FF compared to healthy subjects. The wide range of FF may be due to the treatment given does not equally reduce body haematopoietic needs in all patients.Acknowledgements
We thank Gavin Hamilton (Liver Imaging Group, Department of Radiology, University of California San Diego) for providing technical support and prior knowledge file for spectra analysis. This study was funded by grant RP042C-17AET and BKS050-2017. No other potential conflict of interest relevant to this article was reported.References
- Weatherall DJ. Pathophysiology of thalassaemia. Baillieres Clin Haematol. 1998.
- Turner RT, Martin SA, Iwaniec UT. Metabolic Coupling Between Bone Marrow Adipose Tissue and Hematopoiesis. Curr Osteoporos Rep. 2018.
- Hamilton G, Middleton MS, Bydder M, et al. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging. 2009.
- Vanhamme L, Van Den Boogaart A, Van Huffel S. Improved Method for Accurate and Efficient Quantification of MRS Data with Use of Prior Knowledge. J Magn Reson. 1997.
- Naressi A, Couturier C, Castang I, De Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001.
- Wilson A, Fu H, Schiffrin M, et al. Lack of adipocytes alters hematopoiesis in lipodystrophic mice. Front Immunol. 2018.
Figures
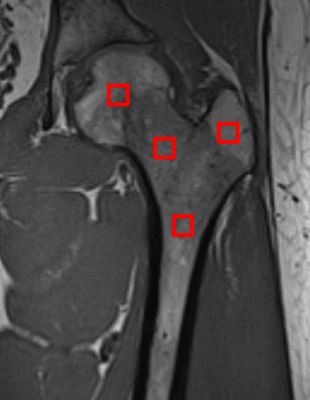
Fig.1 Coronal T1-weighted image of the proximal femur. From left to right (femoral head, femoral neck, diaphysis and greater trochanter).
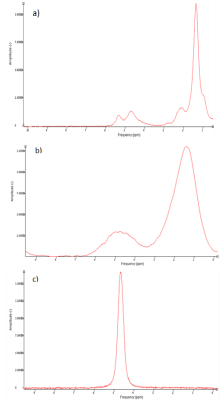
Fig. 2 Examples
of the acquired single voxel STEAM MR Spectra in the diaphysis of three
different subjects: a) healthy(male, 29 years old), b) and c) β-thalassaemia patients (female, 29 years old and male, 29 years old respectively).
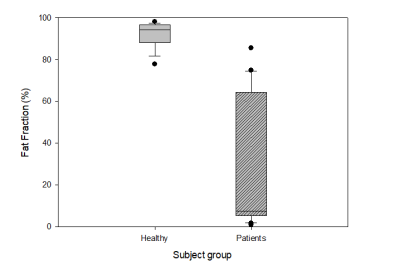
Fig. 3 Boxplot of the fat fraction(%) in bone marrow femur of healthy subjects and β-thalassaemia patients.