2752
Prospective clinical validation of 3D MP2RAGE for T1 mapping of hip cartilage: Preliminary results in patients with hip pain1Department of Diagnostic, Interventional and Pediatric Radiology, University of Bern, Inselspital Bern, Bern, Switzerland, 2Department of Orthopaedic Surgery, Harvard Medical School, Boston Children`s Hospital, Boston, MA, United States, 3Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 4Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 6Department of Radiology, Harvard Medical School, Boston Children`s Hospital, Boston, MA, United States
Synopsis
Current standard techniques for T1 mapping of hip cartilage are affected by flip angle variations due to B1-field inhomogeneities at 3T. The aim was to prospectively compare a 3D MP2RAGE for T1 mapping of cartilage to a 2D-IR based reference standard in the patients with hip pain undergoing indirect MR arthrography. 3D MP2RAGE correlated well with the 2D-IR reference standard and showed a minor systematic bias. This supports the continued use of 3D MP2RAGE in patients undergoing joint preserving hip surgery with the goal to establish prognostic predictors to predict the success or failure of these interventions.
Introduction
The recognition of minor osseous abnormalities of the hip such as femoroacetabular impingement deformities or developmental dysplasia of the hip and their association with hip pain and early osteoarthritis along with the development of new surgical techniques has led to an exponential number of hip preserving surgeries performed in the past 10 years1. It has been shown that the status of preoperative cartilage degeneration determines the long-term outcome of joint preserving procedures2. However standard morphologic MRI of the hip remains elusive in diagnosing early joint degeneration and is limited in its predictive value3. To overcome this limitation, quantitative methods for biochemical assessment of cartilage integrity such as delayed gadolinium enhanced MRI of cartilage have been developed4. T1 mapping of hip cartilage based on a dual-flip angle acquisition following intra-venous administration has shown clinical utility in identifying those patients who benefit from corrective hip surgery at 1.5 T5. However, these results have yet to be reproduced at 3T at which B1-related flip angle variations impair the ability to reliably detect early cartilage degeneration6. By contrast, a magnetization-prepared rapid gradient echo technique with different inversion times (MP2RAGE) has been recently introduced for a B1 insensitive 3D T1 mapping of the brain at higher field strenghts7. Our aim was to apply the 3D MP2RAGE technique and compare it to a 2D-IR based reference standard in a cohort of symptomatic patients eligible for joint preserving hip surgery undergoing indirect MR arthrography at 3T at our institution.Methods
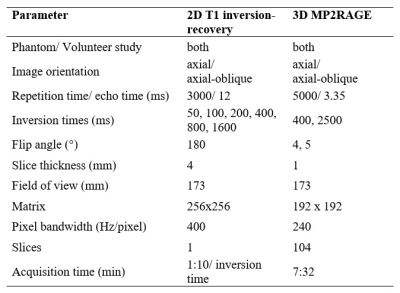
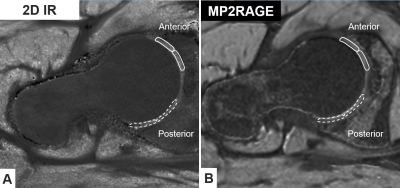
We report on initial 9 patients with hip pain (5 men, 4 women; median age 26 years) undergoing a prospective comparison of 3D MP2RAGE for T1 mapping of hip cartilage to a 2D-IR reference at 3 T (MAGNETOM Skyra, Siemens Healthcare, Germany). All patients had no or only mild radiographic signs of joint degeneration and were diagnosed with hip impingement (5 patients with mixed Cam and pincer FAI) or hip instability due to developmental dysplasia of the hip (4 hips). All patients underwent indirect MR arthrography with a double-dose injection of macrocyclic of gadolinium contrast agent (0.4 ml/kg, 0.2 mmol Gd/kg; Gd-DOTA-, Dotarem, Guerbet, Switzerland). We used Bloch equation simulations7 to optimize the accuracy of T1 measurements within a range of expected cartilage pre- and postcontrast T1-values between 400 and 1100 ms. Our simulations showed optimal inversion times of 400 and 2500ms, with flip angles of 4 and 5 degrees for the GRE readouts. Phase encoding direction was switched to the inner loop of the gradient echo block to enable GRAPPA acceleration in the inner loop to reduce B1 sensitivity by decreasing the number of excitations per TR and to reduce to minimum inversion time to achieve the desired 400ms first inversion time. Sequence parameters are summarized in Figure 1. Image acquisition was performed in the axial-oblique plane for both techniques. As the gold standard T1 mapping technique, a single slice 2D-IR fast spin echo sequence was chosen. For the single slice 2D-IR technique a mid axial-oblique was chosen. The same slice was identified on the 3D MP2RAGE sequence. Then peripheral and central regions of interests were manually placed in the anterior and posterior regions of the weight-bearing femoroacetabular cartilage (Fig. 2).Results
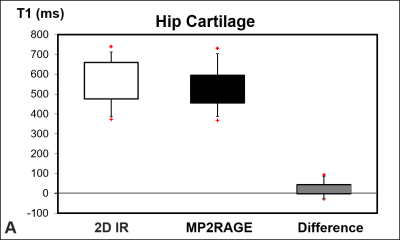
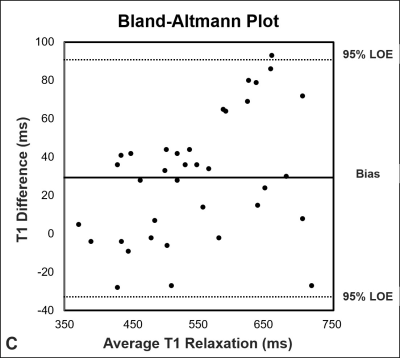
Mean T1 was higher (p < 0.001) for the 2D IR technique (560 ms ± 103 ms) compared to the 3D MP2RAGE (531 ms ± 90 ms) (Fig. 3A). T1 values ranged from 374 ms to 740 ms for the 2D-IR acquisition and ranged from 369 ms to 731 ms for the MP2RAGE technique. Correlation between the 2D IR- and the 3D MP2RAGE was almost perfect rp= 0.95 (p < 0.001) (Fig. 3B). Bland-Altmann analysis showed a bias of 29 ms with 95% level of agreement of -35 ms to 93 ms (Fig. 3C).Discussion
To the best of our knowledge this is the first study to use a 3D-IR based technique such as 3D MP2RAGE for T1 mapping of hip cartilage in young patients with hip pain. Despite the small number of subjects in this initial analysis, we found encouraging results in this first clinical validation of the MP2RAGE sequence. MP2RAGE produced high-resolution T1 maps in all three planes. We observed a bias of 29 ms between the 3D MP2RAGE compared to the 2D-IR reference standard. This bias is less than the clinically relevant range of 50ms to 100 ms, which reportedly reflects different stages of morphologic cartilage damage and thus the observed bias can be considered negligible8. In summary this preliminary report supports the use of the 3D MP2RAGE technique for T1 mapping of hip cartilage in a clinical setting with the goal to establish the prognostic role of delayed gadolinium enhanced MRI of cartilage and improve surgical decision making in joint preserving hip surgery.Acknowledgements
No acknowledgement found.References
1. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA: Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003:112–120.
2. Steppacher SD, Anwander H, Zurmühle CA, Tannast M, Siebenrock KA: Eighty percent of patients with surgical hip dislocation for femoroacetabular impingement have a good clinical result without osteoarthritis progression at 10 years. Clin Orthop Relat Res 2015; 473:1333–1341.
3. Pfirrmann CWA, Duc SR, Zanetti M, Dora C, Hodler J: MR arthrography of acetabular cartilage delamination in femoroacetabular cam impingement. Radiology 2008; 249:236–241.
4. Kim Y-J, Jaramillo D, Millis MB, Gray ML, Burstein D: Assessment of early osteoarthritis in hip dysplasia with delayed gadolinium-enhanced magnetic resonance imaging of cartilage. J Bone Joint Surg Am 2003; 85-A:1987–1992.
5. Kim SD, Jessel R, Zurakowski D, Millis MB, Kim Y-J: Anterior delayed gadolinium-enhanced MRI of cartilage values predict joint failure after periacetabular osteotomy. Clin Orthop Relat Res 2012; 470:3332–3341.
6. Riley GM, McWalter EJ, Stevens KJ, Safran MR, Lattanzi R, Gold GE: MRI of the hip for the evaluation of femoroacetabular impingement; past, present, and future. J Magn Reson Imaging 2015; 41:558–572.
7. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele P-F, Gruetter R: MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010; 49:1271–1281.
8. Bulat E, Bixby SD, Siversson C, Kalish LA, Warfield SK, Kim Y-J: Planar dGEMRIC Maps May Aid Imaging Assessment of Cartilage Damage in Femoroacetabular Impingement. Clin Orthop Relat Res 2016; 474:467–478.
Figures