2751
Tibial Rotation and Knee Flexion Moment Correlate to Patellofemoral Deep Cartilage UTE-T2* 2 Years After ACL Reconstruction1Orthopaedic Surgery, Stanford University, Stanford, CA, United States, 2Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, United States, 3Mechanical Engineering, Stanford University, Stanford, CA, United States
Synopsis
Patellofemoral joint osteoarthritis (PFOA) following anterior cruciate ligament reconstruction (ACLR) is thought to arise, in part, due to increased external rotation of the tibia and decreased quadriceps strength that alter the tracking of the patella in the trochlear groove. In this study of 59 subjects 2 years after ACLR, higher cartilage UTE-T2* values were detected in ACLR knees with greater external tibial rotations and greater knee flexion moments assessed by gait analysis. This study provides evidence that UTE-T2* is sensitive to patellofemoral cartilage degeneration likely due to altered patellar tracking and quadriceps strength in knees at risk of PFOA.
Introduction
Patellofemoral joint osteoarthritis (PFOA) is unfortunately common after anterior cruciate ligament reconstruction (ACLR), with a median prevalence of approximately 50% 10-15 years after surgery1. PFOA following ACLR is thought to arise, in part, due to increased external rotation of the tibia and decreased quadriceps strength that alter the alignment and tracking of the patella in the trochlear groove1, 2. An examination of cartilage thickness changes in the patellofemoral joint demonstrated significant loss of cartilage to both trochlear and patellar cartilage over 5 years following ACLR, with the largest losses observed within the first 2 years of surgery3. Previous ultra-short echo time (UTE-T2*) mapping assessments of deep cartilage collagen matrix integrity and organization have demonstrated cartilage degeneration as evidenced by elevated UTE-T2* in medial tibiofemoral cartilage of nearly half of the ACLR subjects examined at 2 years post-reconstruction4, 5. The goal of this study is to determine if greater external rotation of the tibia or lower knee flexion moment (as an indicator of reduced quadriceps strength) is associated with greater cartilage UTE-T2* at 2 years after ACLR.Methods
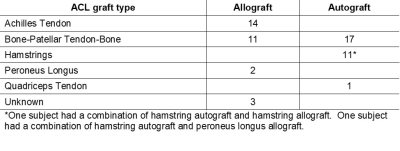
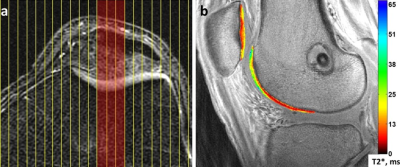
Fifty-nine ACLR subjects (31 males; age 33±11yrs; BMI 25±3 kg/m2; 2.1±0.2yrs post-ACLR) and 20 uninjured controls (11 males; age 29±9yrs, BMI 22±3 kg/m2) participated in these IRB-approved studies, undergoing 3T MRI (GE Healthcare) with an 8-channel knee coil. UTE-T2* maps were calculated via mono-exponential fitting of a series of T2*-weighted MR images acquired at eight TEs (32μs -16ms, non-uniform echo spacing) using a radial out 3-D Cones acquisition6. Deep articular cartilage (extending from bone-cartilage interface through half the cartilage thickness)7, was manually segmented in 2 “tread mark” regions on patellar and trochlear surfaces. The tread marks were 9mm wide (medial to lateral), centered on the patella and on the opposing surface of the trochlea, Figure 1. UTE-T2* maps and mean values were calculated with MRIMapper (MIT) and MATLAB (TheMathWorks). ACLR subjects underwent gait analysis, walking at their normal self-selected speed. A 10-camera optoelectronic system (Qualisys, SE) and a force plate (Bertec, OH) were used to measure subjects’ motion at 120Hz. Knee kinematics and kinetics were calculated using BioMove software (Stanford University) and the point cluster technique8. Side-to-side differences (ACLR limb – contralateral limb) in external tibial rotation angle at heel strike (ExtRot) and maximum knee flexion moment (KFM) were compared to UTE-T2* means using Pearson correlations (or Spearman’s rho for non-normally distributed data). Effects of graft type (Table 1), gender, age and BMI were assessed with stepwise linear regression. UTE-T2* differences between ACLR and uninjured controls (n=20), and ExtRot and KFM differences between ACLR limbs and uninjured control limbs from a separate historical data set matched for age, BMI and gender (n=59), were assessed with t-tests (or Mann-Whitney U tests for non-normal distributions). Statistical analyses were performed with SPSS (IBM) and Excel (Microsoft).Results
Side-to-side differences in ACLR subjects’ ExtRot significantly correlated to UTE-T2* in deep patellar cartilage (n=59, R=0.37, p=0.004) where greater ExtRot in the ACLR limb was associated with greater UTE-T2*, Figure 2. Side-to-side differences in ACLR subjects’ KFM significantly correlated to UTE-T2* in deep trochlear cartilage (n=59, R=0.28, p=0.035) where greater KFM in the ACLR limb was associated with greater UTE-T2*. No other significant associations were detected. Stepwise linear regression found no effects of graft type, gender, age or BMI on these results. Deep patellar UTE-T2* in ACLR subjects were 12% higher than in uninjured controls (p=0.020), but deep trochlear UTE-T2* did not differ between cohorts (p>0.19). Compared to uninjured controls from the historical data set (n=59), ExtRot of the ACLR limb was 30% higher than in matched controls (p=0.050), and there was a trend for 16% higher KFM in the ACLR limb compared to matched controls (p=0.091).Discussion
Greater UTE-T2* was detected in ACLR knees with greater ExtRot, supporting the idea that alteration of patellar alignment and tracking due to increased external tibial rotation is detrimental to patellar cartilage health1, 2. A similarly positive correlation detected between side-to-side differences in KFM and trochlear cartilage UTE-T2* suggests that those who are engaging their quadriceps more in this cohort show signs of cartilage stress. This finding is consistent with previous studies showing greater KFM to be associated with medial tibiofemoral cartilage thickness losses over 5 years in established OA patients9 and that ACLR subjects with greater KFM at 2 years after surgery reported worse KOOS pain and quality of life scores at 8 years follow-up10. A lack of association between ExtRot and trochlear cartilage UTE-T2*, the weak correlation between KFM and trochlear UTE-T2*, and the lack of trochlear UTE-T2* differences between controls and ACLR subjects may result from the large size of trochlear tread mark regions and their widely-ranging UTE-T2*, Figure 1. Effects of focal UTE-T2* degeneration within trochlear tread marks may have been washed out due to averaging over such large regions. Future work will seek to determine if a smaller sub-region of the trochlea is more sensitive to ACLR gait alterations in these subjects.Conclusion
This study provides evidence that UTE-T2* is sensitive to patellofemoral cartilage degeneration likely due to altered patellar tracking and quadriceps strength at 2 years after ACLR in knees at risk of PFOA.Acknowledgements
NIH RO1 AR052784 (PI Chu) and GE Healthcare for MRI scan time and sequence supportReferences
1. Culvenor AG, Cook JL, Collins NJ, Crossley KM. Is patellofemoral joint osteoarthritis an under-recognised outcome of anterior cruciate ligament reconstruction? A narrative literature review. Br J Sports Med. 2013 Jan;47(2):66-70.
2. Van de Velde SK, Gill TJ, DeFrate LE, Papannagari R, Li G. The effect of anterior cruciate ligament deficiency and reconstruction on the patellofemoral joint. Am J Sports Med. 2008 Jun;36(6):1150-9.
3. Culvenor AG, Eckstein F, Wirth W, Lohmander LS, Frobell R. Loss of patellofemoral cartilage thickness over 5 years following ACL injury depends on the initial treatment strategy: results from the KANON trial. Br J Sports Med. 2019 Sep;53(18):1168-73.
4. Chu CR, Williams AA, West RV, Qian Y, Fu FH, Do BH, et al. Quantitative Magnetic Resonance Imaging UTE-T2* Mapping of Cartilage and Meniscus Healing After Anatomic Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2014 Aug;42(8):1847-56.
5. Williams AA, Titchenal MR, Do BH, Guha A, Chu CR. MRI UTE-T2* Shows High Incidence of Cartilage Subsurface Matrix Changes 2 Years After ACL Reconstruction. J Orthop Res. 2019 Feb;37(2):370-377.
6. Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006 Mar;55(3):575-82.
7. Williams A, Qian Y, Chu CR. UTE-T2 * mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthritis Cartilage. 2011 Jan;19(1):84-8. Epub 2010/11/03.
8. Dyrby CO, Andriacchi TP. Secondary motions of the knee during weight bearing and non-weight bearing activities. J Orthop Res. 2004 Jul;22(4):794-800.
9. Chehab EF, Favre J, Erhart-Hledik JC, Andriacchi TP. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthritis Cartilage. 2014 Nov;22(11):1833-9.
10. Erhart-Hledik JC, Chu CR, Asay JL, Andriacchi TP. Gait mechanics 2 years after anterior cruciate ligament reconstruction are associated with longer-term changes in patient-reported outcomes. J Orthop Res. 2017 Mar;35(3):634-40.
Figures