2745
Accelerated T1ρ Acquisition for Knee Cartilage Using Compressed Sensing:Quantitative evaluation1Shandong Medical Imaging Research Institute,Shandong University, Jinan, China, 2Philips Healthcare, Shanghai, China
Synopsis
The current work evaluates the influence of compressed sensing accelerated imaging on the T1ρ mapping. Quantitative evaluation is done using quantitative T1ρ mapping for knee cartilage quantification. T1ρ quantification after CS-accelerated acquisition was compared with non-CS-accelerated acquisition for central patella cartilage.
Introduction
Quantitative T1ρ mapping has been used as a novel MRI sequence for many biomedical applications, including neurodegenerative, cardiovascular, body, and musculoskeletal imaging to assess early macromolecular changes rather than conventional morphological imaging1,2,3 .Quantitative T1ρ imaging requires the acquisition of multiple images with different spin-lock times (TSLs) to generate the T1ρ maps. This translates into long scan times, thus limiting the widespread clinical use of this technique4,5. Thus, the goal of this study was to evaluate the accuracy of accelerated T1ρ mapping for knee cartilage quantification.Materials and Methods
Compressed sensing (CS) is still a new technique for accelerating image acquisition. It relies on the inherent sparsity and compressibility of MR data to overcome undersampling-induced artifacts in the acquired data6,7. Keeping good image quality as acceleration factor (AF) increases is extremely important for rapidly obtaining good relaxation maps8. Successful CS acceleration is obtained when the incoherently measured data are accurate9. A sequential combination of CS, during data acquisition with different acceleration factor (AF) was used to accelerate the acquisition of T1ρ maps. Quantitative evaluation: T1ρ maps acquired at different CS-accelerated factors by 2-8 were computed and mean T1ρ values of central patella cartilage were statistically compared using the ANOVA test. After obtaining informed consent, eight adult healthy volunteers were imaged for this study. T1ρ quantification after non CS-accelerated acquisition(NCS-T1ρ)served as reference from CS-accelerated quantification(CS-T1ρ). Imaging was performed on a 3.0T Philips ingenia CX system using the T1ρ sequence. Figure 1 shows sequence parameters of T1ρ mapping with different acceleration factors.Results
Figure 2 shows NCS-T1ρ and various CS-T1ρ images from one of the volunteers. Intra- cartilage contrast is visually similar between the images. Figure3 and Figure4 shows the mean T1ρ values along with the corresponding standard deviations from eight subjects. Statistically, measurements from the NCS-T1ρ and CS-T1ρ with different AF were found to be statistical difference (p<0.05) in all the subjects. However, there was no significant difference of T1ρ values between the NCS-T1ρ and the CS-T1ρ in AF of 2 and 8.Discussion
CS-T1ρ MR imaging provides equivalent image quality compared with corresponding NCS-T1ρ MR imaging in volunteers. CS acceleration does not appear to adversely impact overall image quality relative to the corresponding conventional acquisitions. The increasing standard deviation in T1ρ maps with increasing AF except for AF=8. It is consistent with CS algorithms where incoherent aliasing increases with increasing factors.CS-T1ρ with an acceleration factor of 2 was not influenced T1ρ values significantly when compared with the NCS-T1ρ acquisition. Limitations: The clinical cohort evaluated was relatively limited in size. Additionally, current study evaluated imaging acceleration using CS in healthy volunteers. Individuals with osteoarthritis were not included in our clinical cohort. Furthermore, the sample size in evaluation was small. Future studies will focus on evaluation in early osteoarthritis with larger sample size, along with comparison with accelerated T1ρ mapping.Conclusion
Accelerated T1ρ mapping of cartilage with CS is feasible up to measure knee cartilage. Furthermore, mean T1ρ values were not influenced by CS ,up to AF 2. These results show that this technique holds great promise in making T1ρmapping more accessible for clinical applications.Acknowledgements
No acknowledgement found.References
1. Haris M, Singh A, Cai K, et al. T1ρ MRI in Alzheimer’s disease: detection of pathological changes in medial temporal lobe. J Neuroimaging. 2011;21:e86-e90.
2. Wang L, Regatte RR. T1q MRI of human musculoskeletal system. J Magn Reson Imaging. 2015;41:586-600.
3. Zibetti MVW,Sharafi A.Accelerating 3D-T mapping of cartilage using compressed sensing with different sparse and low rank models.Magn Reson Med.2018 80(4):1475-1491.
4. Pandit P,Rivoire J,Accelerated T1ρ acquisition for knee cartilage quantification using compressed sensing and data-driven parallel imaging: A feasibility study.Magn Reson Med.2016 75(3):1256-61
5. Zhao F, Deng M, Yuan J, Teng G-J, Ahuja AT, Wang Y-XJ. Experimental evaluation of accelerated T1rho relaxation quantification in human liver using limited spin-lock times. Korean J Radiol 2012;13: 736–742.
6. Lustig M, Donoho D, Pauly JM. Sparse MRI: the application of compressed sensing for rapid MR imaging. Magn Reson Med 2007;58: 1182–1195.
7. Liang D, Liu B, Wang J, Ying L. Accelerating SENSE using compressed sensing. Magn Reson Med 2009;62:1574–1584.
8. Lustig M, Donoho DL, Santos JM, Pauly JM. Compressed sensing MRI. IEEE Signal Process Mag 2008;25:72–82.
9. Vasanawala SS, Murphy MJ, Alley MT, Lai P, Keutzer K, Pauly JM, Lustig M. Practical parallel imaging compressed sensing MRI: summary of two years of experience in accelerating body MRI of pediatric patients. In: 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, Illinois, USA, March 30 to April 2, 2011. p 1039–1043.
Figures
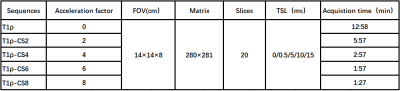
Figure1:Table shows sequence parameters of T1ρ mapping with different acceleration factors.
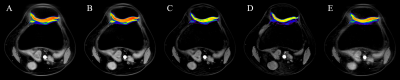
Figure2:T1ρ color maps of the central patella measured in one of the volunteers. A-non CS-accelerated T1ρ , B- CS-accelerated T1ρ(AF=2) ,C- CS-accelerated T1ρ(AF=4),D- CS-accelerated T1ρ(AF=6) E- CS-accelerated T1ρ(AF=8)
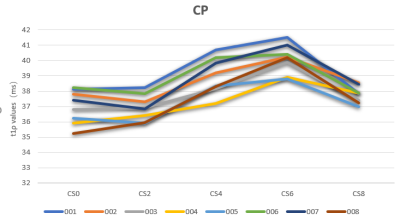
Figure3:The mean T1ρ values of different compressed sensing (CS) acceleration factors(AF) in the central patella.CP-central patella,CS2, CS4, CS6, CS8, CS10 represent AF=0,2,4,6,8 respectively.
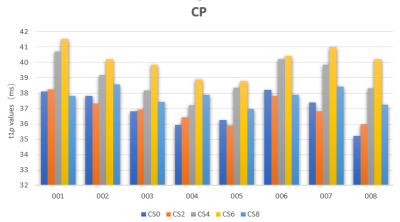
Figure4: The mean T1ρ values of eight volunteers in the central patella. .CP-central patella. CS2, CS4, CS6, CS8, CS10 represent AF=0,2,4,6,8 respectively.