2740
Site-Specific Quantitative µMRI and Polarized Light Microscopy (PLM) Study of Young Rabbit Femur Cartilage
Yang Xia1, Syeda Batool1, and Mohammad Hammimi1
1Physics, Oakland University, Rochester, MI, United States
1Physics, Oakland University, Rochester, MI, United States
Synopsis
This work aims to characterize the site-specific and depth dependent molecular and morphological structures in young rabbit femur cartilage, using quantitative µMRI (T2 relaxation) and Polarized light microscopy (PLM).
Introduction
Articular cartilage (AC) is a thin layer of protective tissue covering the articular ends of bones in diarthrodial joints. It is a load-bearing tissue with complex composition and architecture. The molecular composition of AC mainly are water, proteoglycans (PGs), and collagen network. The architecture of AC is dominated by its collagen network, which has a depth-dependent organization. In mature cartilage, an arcade-like fibril structure exists, with a 90˚ orientation difference between the surface fibers and the deep fibers. It has been found that arcade-like structure is absent in juvenile or inborn cartilage.Material and methods
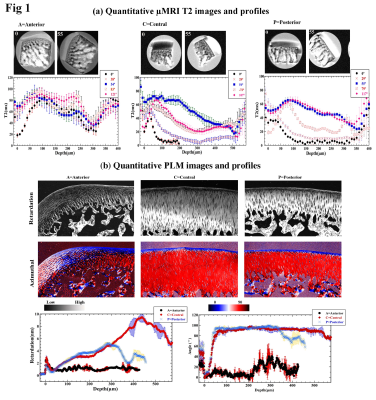
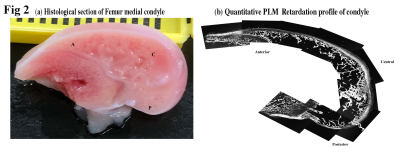
A number of 12-14 weeks old White New Zealand male rabbits were used in this study. 3 different sample sites were chosen on femoral medial condyle (anterior, central and posterior). One cartilage-bone plug (~2.0 mm thick) was harvested from each location, with the full thickness of cartilage still attached to underlying bone. Quantitative T2 mapping of the specimens was carried out at various orientations in B0 using a magnetization-prepared 2D spin-echo sequence at slice thickness 0.8 mm and at 9.75 µm/pixel resolution. After µMRI, 6.0 µm thin sections were cut from each sample to generate quantitative PLM images at 1 µm resolution. In addition, thin histological sections that spanned the entire central femoral medial condyles (Fig 1a) were imaged at 4.0 µm/pixel resolution, to study the heterogeneity of the collagen fibril organization across the tissue in both horizontal and vertical directions.Results
We found significant topographical variations in both cartilage thickness and its collagen organization across the joint surface at different anatomical sites.µMRI Results: AC in T2-weighted images of medial central and medial posterior samples appeared bilaminar at the 0˚ and its corresponding T2 profiles had an asymmetrical bell-shaped curve that resembles the classical three-zone structure in the tissue (Fig 1a). AC in T2-weighted image of anterior site appeared less laminated at 0° and its T2 profile was broader and symmetrical along the depth. The T2 anisotropy effect was smaller in the anterior site as compared to central and posterior sites.
PLM results: The medial central and medial posterior sites have developed the arcade like structure but the anterior sites are not - the collagen fibers there run predominantly parallel to articular surface and there is increased cellular density at the anterior site (Fig 1b). The quantitative retardation and azimuthal profiles for all three sites has been obtained and compared. Overall, the lowest retardation values (which indicate the lowest mutual organization among the collagen fibers) have been found in the medial anterior site, with the predominant parallel fibers w-r-t to articular surface. The PLM results from the entire central femoral medial condyles (Fig 2) clearly support the µMRI and PLM results from the individual blocks.