2733
Application of Compressed Sensing to Quantitative Double Echo steady state (qDESS) for Rapid T2 Relaxometry in Knees1Clinical Science, Philips Healthcare NA, San Francisco, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States
Synopsis
Quantitative Double Echo
Steady State (qDESS) is a fast 3D MRI sequence capable of simultaneous knee
morphometry and T2 relaxometry. Compressed-sensing based acceleration methods are
more and more readily available from major MRI vendors, and have the potential to
further speed up qDESS and to provide greater clinical value. In this study, we
investigate the feasibility of applying Philips Compressed SENSE (CS) to qDESS.
We show that CS does not bias the T2 accuracy of qDESS. Our findings support
the clinical application of CS accelerated qDESS for fast and quantitative MR
knee assessment.
INTRODUCTION
It has been shown that cartilage and meniscal T2 are promising imaging markers for the diagnosis and treatment of Osteoarthritis (OA), the leading cause for disability in older population.1,2 3D quantitative double echo steady state (qDESS) offers a rapid five-minute knee MRI scan, capable of simultaneous 3D morphometry and T2 relaxometry for comprehensive whole knee assessment.3 To promote standardization and clinical adoption of qDESS, we previously compared the performance of the sequence across vendors and showed good reliability and reproducibility in healthy human volunteers.4 Recently, compressed-sensing-based acceleration techniques have become available from all major MRI vendors.5,6,7 In particular, Philips’ Compressed SENSE (CS) combines compressed sensing and parallel imaging and is compatible with 3D qDESS. While parallel imaging has commonly been used to accelerate qDESS, application of CS to qDESS has not been validated. In this study, we compare the performance of fully-sampled qDESS with those accelerated by conventional parallel imaging (SENSE) and CS in healthy human knees.METHODS
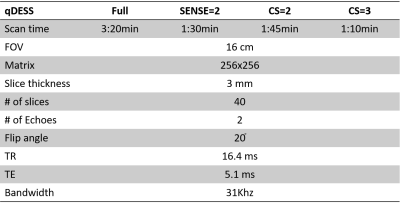
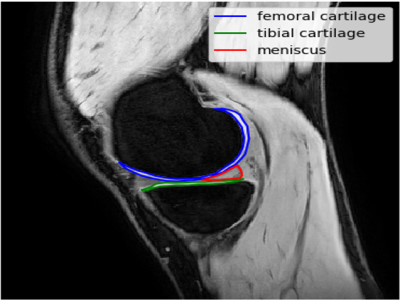
Both left and right knees of three healthy volunteers (35-65yrs) were scanned individually on a Philips Ingenia 3T scanner (Philips, Netherlands) using a 16channel T/R knee coil and qDESS sequence4. Four prospectively acquired qDESS scans were compared: fully sampled qDESS, SENSE=2 (in anterior posterior phase encoding direction), CS=2, and CS=3, respectively. The CS scans used vendor implemented 3D variable-density k-space undersampling and online reconstruction for real-time image production on the scanner. Table 1 shows the scan times and sequence parameters.T2 quantification was performed by inverting the qDESS signal model 8. Medial femoral and tibial cartilage and the posterior horn of the medial meniscus were manually segmented from the first echo qDESS images in the most medial slice of the medial femoral condyle (Figure 1). The reported T2 values were averages of all pixel T2 values in the segmented region of interest (ROI). We used a Friedman test and post hoc Wilcoxon signed rank tests (p<0.05) to detect differences in the measured T2 values between the four qDESS scans. Pearson correlation coefficient was also calculated to assess the relation of the measured T2 values between scans.
RESULTS
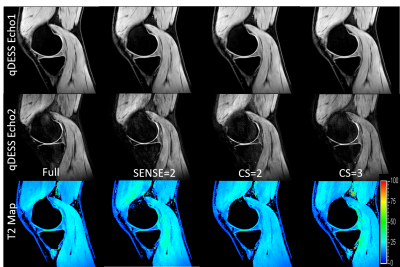
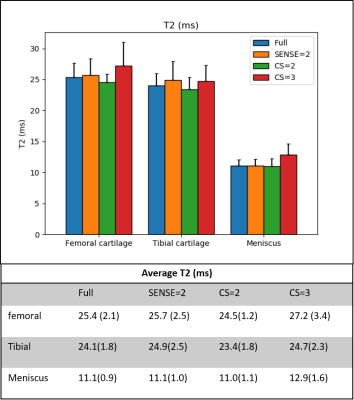
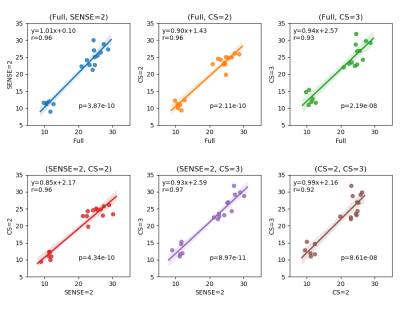
Figure 2 shows representative images of the first and second echoes of qDESS and the calculated T2 maps from a single knee slice in subject 1. No major imaging artifacts were observed in the qDESS images, and the calculated T2 maps were similar within the knee joints.Figure 3 compares the mean T2 values in the femoral cartilage, tibial cartilage and meniscus. The T2 measurements across different qDESS scans were comparable and no statistically significant difference was found (Friedman test p = 0.18). Figure 4 shows that the measured T2 values were highly correlated among all pairs of qDESS scans (r = 0.92 - 0.97) with regression slopes close to unity.
DISCUSSION
SENSE=2 and CS=2 resulted in a 50% reduction, and CS=3 resulted in a 70% reduction in scan time compared to fully sampled qDESS. Such accelerations could provide multiple clinical benefits, such as reduced sensitivity to motion, reduction in sedation time and increased patient throughputs. No major artifacts were observed in qDESS images. Subtle and localized differences in noise patterns could be discerned among the SENSE and CS scans by adjusting the windowing level, especially in the 2nd echo images where signal-to-noise ratio was lower. These subtle differences were not unexpected as the aliasing patterns of SENSE and CS are known to be different. Additionally, noise thresholding used in CS reconstruction produces an inherent de-noising effect which could be perceived as an advantage of CS over SENSE. The de-noising effect may explain the similar image appearance between CS=3 and SENSE=2 scans despite the higher acceleration factor in CS than in SENSE.Further investigations are merited to better characterize the impact of CS acceleration on image quality. Nevertheless, we found good agreement in the measured T2 values of femoral cartilage, tibial cartilage and meniscus across all scans, indicating that the acceleration methods used in this study were adequate for robust T2 relaxometry. Future incorporation of deep-learning-based segmentation tools9.10 will help reduce the variances associated with the manual segmentation employed in the current study. Similar comparisons in pathological cases will also be beneficial to further validate the clinical utility of CS accelerated qDESS.
CONCLUSION
In this study, we compared the effect of SENSE and CS to qDESS in healthy volunteers’ knees, and found good agreement in the estimated T2 values between fully sampled qDESS and qDESS with SENSE and CS. Our findings suggests the feasibility of applying CS to qDESS for fast and quantitative MR knee assessment.Acknowledgements
This study was supported by Philips Healthcare and NIH R01 AR0063643.References
1. Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage. 2013;21:1474–84
2. Eckstein, Felix, et al. Imaging research results from the Osteoarthritis Initiative (OAI): a review and lessons learned 10 years after start of enrolment. Annals of the rheumatic diseases. 2014;73(7): 1289-1300.
3. Chaudhari AS. et al. Combined 5‐minute double‐echo in steady‐state with separated echoes and 2‐minute proton‐density‐weighted 2D FSE sequence for comprehensive whole‐joint knee MRI assessment. Journal of Magnetic Resonance Imaging. 2019;49(7): e183-e194.
4. Lu Q, Hargreaves BA, Hitt D, Chaudhari AS, Knee T2 relaxometry using quantitative DESS: reproducibility across imaging vendors. Proceedings of the International Society of Magnetic Resonance in Medicine 27th Annual Meeting and Exhibition. 2019; p4473.
5. Kijowski R, Rosas H, Samsonov A, King K, Peters R, Liu. F. Knee imaging: three dimenstional fast spin echo sequence with compressed sensing, Magn Reson Imaging: 2017;45(6):1712-1722
6. Henninger B, Raithel E, Kranewitter C, Steurer M, Jaschke W, Kremser C. Evaluation of an accelerated 3D SPACE sequence with compressed sensing and free stop scan mode for imaging of the knee. Eur J Radiol. 2018;102:74-82
7. Sartoretti T et al. Reduction of procedure times in routine clinical practice with Compressed SENSE magnetic resonance imaging technique. PLoS ONE. 2019;14(4):e0215887
8. Sveinsson B, Chaudhari AS, Gold GE, Hargreaves BA. A simple analytic method for estimating T2 in the knee from DESS. Magn Reson Imaging. 2016;38:63-70.
9. Liu F, Zhou Z, Jang H, Samsonov A, Zhao G, Kijowski R. Deep convolutional neural network and 3D deformable approach for tissue segmentation in musculoskeletal magnetic resonance imaging. Magn Reson Med 2018;79:2379-2391.
10. Norman B, Pedoia V, Majumdar S. Use of 2D U-Net Convolutional Neural Networks for Automated Cartilage and Meniscus Segmentation of Knee MR Imaging Data to Determine Relaxometry and Morphometry. Radiology 2018;288(1):177-185.
Figures