2727
Diffusion tensor imaging of articular cartilage at 3T: repeatability and sensitivity to degenerative changes1Department of Radiology, Stanford University, Stanford, CA, United States
Synopsis
Mean Diffusivity (MD) and Fractional Anisotropy (FA) derived from DTI are a very promising biomarker of cartilage health. Many current implementations of DTI require high field scanners, and its feasibility utility at 3T is unclear. The aim of this study was to develop an optimized DTI acquisition and postprocessing protocol for the evaluation of femoral articular cartilage at 3T in a clinically feasible scan time, and to assess its repeatability and sensitivity to transient changes induced by exercise. Our results show that MD and FA measurements at 3T are repeatable and robust to changes induced by exercise.
Introduction
Osteoarthritis (OA) is one of the leading causes of disability in industrialized countries and represents a dramatically increasing financial burden. Diffusion Tensor Imaging (DTI) is an MRI-based technique that provides information on water mobility and has been proposed as a biomarker in OA due to its sensitivity to both collagen organization (through the Fractional Anisotropy (FA)) and proteoglycan content (through the Mean Diffusivity (MD)), and its reduced sensitivity to dipolar interactions(1).However, current implementations of DTI in articular cartilage require higher field strength or long acquisition times, which limit its clinical applications and use in clinical trials for disease modifying treatments in OA.
The aim of this study was to develop an optimized DTI acquisition and postprocessing protocol for the evaluation of femoral articular cartilage at 3T in a clinically feasible scan time, and to assess its repeatability and sensitivity to transient changes induced by exercise.
Methods
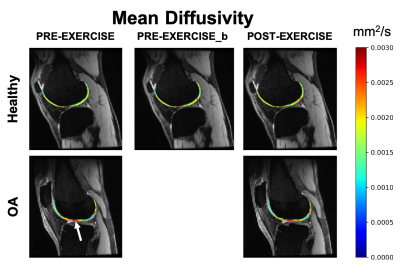
Osteoarthritic subjects(n=12, BMI=27.5, age=61 y/o) and healthy controls(n=3, BMI=24.5, age=34 y/o) were included in this study. For all the subjects, both knees were scanned on a GE 3T scanner (GE Healthcare, Milwaukee, WI, USA) using two 16-channel flexible phased-array, receive-only coils (NeoCoil, Pewaukee, WI, USA). A bilateral(2) 3D quantitative double-echo in steady-state (qDESS) sequence was used for anatomical reference and for computation of T2(3).Two DWI-EPI scans were used to scan both knees separately (TE/TR=67/4000 ms, single-shot EPI readout, FOV=16x16x84 mm3, matrix size=128x128x22, 3 b0 scans, spectral-spatial water excitation, b=400 mm2/s, 15 diffusion directions acquired twice). The total acquisition time was 3min40s for each knee.Each subject was asked to evaluate their pain level (using a NPRS scale) for the left and right knee separately. After the first baseline scan (“PRE-EXERCISE”), all patients and healthy subjects were asked to perform a one-legged squat until exhaustion with the most painful knee, and the scan was repeated (“POST-EXERCISE”). During the exercise, performed outside the scanner, the non-exercise leg was unloaded.
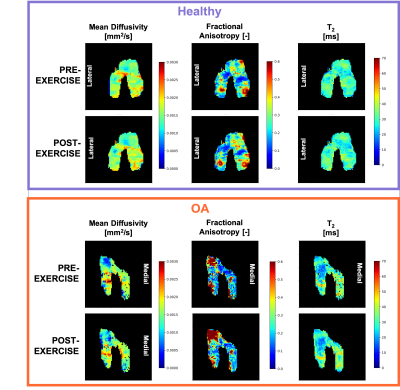
For the control subjects, the full MRI protocol was repeated ("PRE-EXERCISEb") after subject repositioning to evaluate repeatability.Femoral cartilage was automatically segmented on the first echo of the qDESS at baseline using a deep learning algorithm previously described(4) and manually adjusted as needed. The femoral cartilage was then automatically subdivided into 6 compartments (Medial Posterior, Medial Central, Medial Anterior, Lateral Posterior, Lateral Central, Lateral Anterior).
All diffusion images were analyzed with custom python code using Dipy(5). The DWI data was first denoised used a PCA-based denoising algorithm, and all diffusion-weighted images scans were registered to the first non-diffusion-weighted volume and to the baseline qDESS scan to correct for eddy current-induced and EPI-related distortions. The data was fit to a tensor model and MD and FA were calculated.
Regional averages of MD, FA and T2 in OA subjects knee were compared to the corresponding regional averages in healthy subjects with one-way ANOVA. Repeatability and sensitivity to transient effects induced by exercise for T2 and DTI parameters was assessed with Bland-Altman analysis and coefficient of variation (CV).
Results
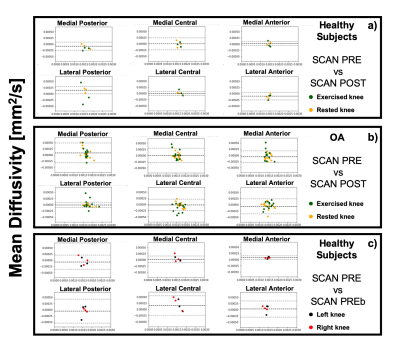
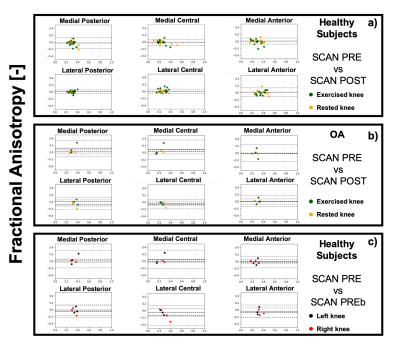
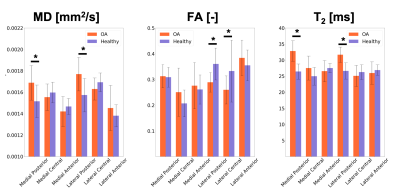
Good scan-rescan repeatability was observer for MD and T2 (CVs ranging from 2.5% and 18%, and from 3% to 11.1% for MD and T2 respectively). Worse scan-rescan repeatability was seen in FA, likely due to its larger sensitivity to noise compared to MD and FA. For the healthy controls, no significant differences were observed related to exercise for any of the evaluated metrics (T2, MD and FA), and the CV for the scans before and after exercise was in the same range as the general scan-rescan repeatability.8 out of 12 OA subjects reported pain in both knees. For these subjects, no significant differences in T2, MD, and FA were observed at baseline between the exercised (n=6 left and n=6 right) and the rested knee. Significant differences(p< 0.05) in MD were detected at baseline between left and right knee for the OA group in the medial central, lateral central and lateral posterior compartment, and in FA for all 6 cartilage compartments.
Similar CVs were found for the OA subjects as for healthy subjects, with no significant effect of exercise on quantitative parameters in articular cartilage.
Discussion
In this work, we demonstrate that DTI of articular cartilage can be obtained in less than 5 minutes at 3T with good repeatability.DTI provides additional metrics to T2 for the evaluation of cartilage microstructure. OA knees present elevated values of MD compared to healthy controls, potentially reflecting the increased water mobility as a consequence of decrease in proteoglycans content. The decrease in FA in OA patients compared to controls could be an indication of loss of organization in the collagen matrix.
Due to its short scan time, the proposed DTI acquisition protocol combined with ad-hoc postprocessing offers potential for clinical applications.
Further research in a larger population will be needed to assess the potential of DTI parameters in addition to T2 in detecting early changes in articular cartilage and to monitor the effect of treatments. Nonetheless, our results indicate that DTI measurements of articular cartilage at 3T are repeatable, relatively insensitive to transient changes induced by exercise and are able to detect local changes in cartilage microstructure between OA patients and healthy controls.
Acknowledgements
No acknowledgement found.References
1) Ferizi et al. Magn Reson Med 2016
2). Kogan et al. Magn Reson Med 2017
3) Desai et al. Zenodo. http://doi.org/10.5281/zenodo.2559549
4) Sveinsson et al., Magn Reson Imaging 2017
5) Garyfallidis et al.: Front Neuroinform 2014
Figures