2721
Quantitative MRI of muscles is different in rheumatoid arthritis patients compared to healthy controls1University of Leeds, Leeds, United Kingdom
Synopsis
Rheumatoid arthritis can present with the loss of muscle mass and a decrease in strength and functional capability. This study demonstrates that Quantitative MRI can detect differences in the muscles of RA patients, whether they are newly diagnosed, in remission or with persistently active disease. Differences in T2, FF and muscle volume were apparent at diagnosis, suggesting muscle changes in RA occur early. Furthermore, despite RA treatment, patients in remission show worse MRI parameters and strength compared to healthy individuals, suggesting the muscles have pathology. This warrants attention in improving the muscle health throughout the spectrum of the RA continuum.
Background:
Rheumatoid arthritis (RA) is a chronic, progressive, autoimmune, inflammatory disease with a prevalence of 0.8% in the UK (1). It mainly affects synovial joints, producing symmetrical arthritis and is characterised by joint pain and stiffness, which if left untreated can lead to irreversible joint damage and deformity. As the condition develops, patients frequently lose their jobs (2), functional ability (3), and their independence (1). As well as joint damage, RA is also associated with altered body composition. It can present with the loss of muscle mass and a decrease in strength and functional capability. This could be due to an inactive lifestyle as a result of the disease, drug-induced myopathies (4) and the activation of the nuclear factor kappa-beta pathway (NF-κB), which triggers metabolic alterations leading to the degradation of muscle tissue. Furthermore, the production of tumour necrosis factor-alpha (TNF-alpha) and other inflammatory cytokines which are critical to the pathogenesis of RA are reported to have catabolic effects on skeletal muscle (5). These combined factors can result in rheumatoid cachexia (RC), which is characterised by the loss of muscle mass (muscle atrophy) and strength, with the preservation, or increase, of fat mass. RC is one of the most common complications of RA. RC has been shown to be associated with increased disease severity outcomes including reduced quality of life, increased fatigue and increased morbidity and mortality and can accelerate age-related sarcopenia.Quantitative MRI offers a non-invasive measurement of muscle status which could improve the understanding of muscle pathology in RA. The purpose of this study was to assess whether MRI-based measurements of T2, fat fraction (FF), diffusion tensor imaging and muscle volume can detect differences between the muscles of RA patients and healthy controls in the thigh; and to assess how different stages of the disease present differently.
Methods:
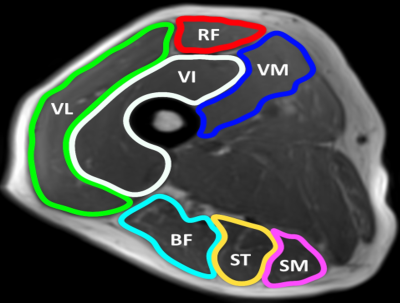
This study was conducted in a cross-sectional design at the Leeds Teaching Hospitals NHS Trust (LTHT). 39 RA patients were recruited, comprised of 3 groups: 13 newly diagnosed treatment naïve (New RA: 10/13 female, mean age 63 years, mean CRP 31.5, mean EMS 71 minutes), 13 in clinical remission DAS28 <2.6 for at least 1 year (Remission RA: 10/13 female, mean age 67 years, mean CRP 12.1, mean disease 74 months, mean EMS 2 minutes), 13 RA with at least 1 year diagnosis, DAS28 >3.2 ± raised CRP/ESR ± DMARD/targeted therapy escalation ± requiring steroid therapy (Resistant RA: 10/13 female, mean age 65, mean CRP 17.4, mean disease 123 months, mean EMS 63 minutes). 13 healthy controls were also recruited. All four groups were age and gender-matched.MR data was acquired using a MAGNETOM Verio 3T MR scanner. Two small four-channel flex coils were wrapped around the dominant thigh and placed with the distal end of both coils positioned 4cm from the superior edge of the patella. Muscle volume, T2, fat fraction and diffusion were measured. Regions of interest were contoured around the individual muscles of the hamstrings and quadriceps. STEAM-EPI imaging sequence was used to measure diffusion along 6 diffusion directions, 2-point Dixon imaging to assess fat fraction and a fat-suppressed turbo-spin echo (TSE) sequence to measure the transverse relaxation time (T2). For T2 measurements, axial images were obtained using a T2 weighted, multi-slice echo (MESE) sequence, with SPAIR fat suppression with an echo train length of 16, and echo times of 9.6, 19.2, 28.8, 38.4, 48.0, 57.6, 67.2, 76.8, 86.4, 96.0, 105.6, 115.2, 124.8, 134.4, 144.0, 153.6ms and a repetition time of 1500ms. All participants had knee extension and flexion power measured using an isokinetic knee extension-flexion (concentric-concentric) dynamometer at 60 degrees a second and handgrip strength measured using an isometric handheld dynamometer. Offline image analysis was performed using MATLAB. Statistical analyses were performed using SPSS. One-Way ANOVA with Bonferroni post hoc analysis was used to test for significant differences. Spearman's rank correlation was used to measure correlation.
Results:
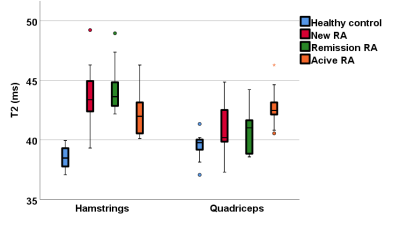
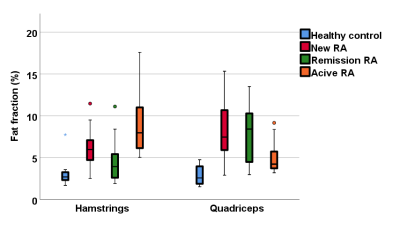
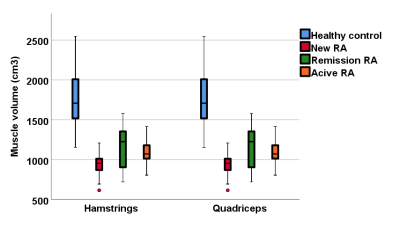
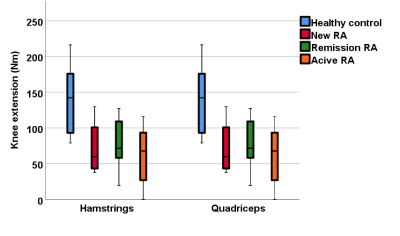
A one-way ANOVA analysis demonstrated significant differences in T2, FF and muscle volume between RA patients and healthy controls, but no difference in MD or FA. There was no significant difference between the RA groups. T2 and FF were higher in RA patients, whilst muscle volume was lower. Muscle volume was significantly correlated with early morning stiffness (rs=0.4, p=0.001), DAS28 (rs=0.4, p=0.001) and grip strength (rs=0.5, p<0.001). All RA patients showed weaker strength compared to the healthy controls. Although the patients in remission had better results compared to new and resistant RA patients, they performed worse than the healthy controls in all strength assessments.Conclusions:
Quantitative MRI can detect significant differences in the muscles of RA patients, whether they are newly diagnosed, in remission or with persistently active disease. Significant differences in T2, FF and muscle volume were apparent even at diagnosis, suggesting muscle changes in RA occur early. Despite effective RA therapy, patients in remission show worse MRI parameters and strength compared to healthy individuals, suggesting that muscle pathology is still present in patients who are deemed in remission. The results of this study demonstrate the importance of improving muscle strength and quality throughout the spectrum of the RA continuum, suggesting it could have importance in clinical practice.Acknowledgements
This paper presents independent research funded/supported by the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. We are grateful to radiographer Dominic Bertham for carrying out the MR studies.References
1) Westhoff, G., J. Listing, and A. Zink, Loss of physical independence in rheumatoid arthritis: interview data from a representative sample of patients in rheumatologic care. Arthritis Care & Research, 2000. 13(1): p. 11-22.
2) Callahan, L. and E. Yelin, The social and economic consequences of rheumatic disease. Primer on the rheumatic diseases, 2001. 12: p. 1-4.
3) Alamanos, Y. and A.A. Drosos, Epidemiology of adult rheumatoid arthritis. Autoimmunity reviews, 2005. 4(3): p. 130-136.
4) Agrawal, V., et al., Muscle involvement in rheumatoid arthritis: clinical and histological characteristics and review of literature. J Indian Rheumatol Assoc, 2003. 11(4): p. 98-103.
5) Schaap, L.A., et al., Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 2009. 64(11): p. 1183-1189.