2719
Measuring Spontaneous Muscular Activities in Neuromuscular Disease: Preliminary Results1Section on Experimental Radiology, University Hospital of Tuebingen, Tuebingen, Germany, 2Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany, 3School of Biomedical Engineering & Imaging Sciences, King's College London, St. Thomas' Hospital, London, United Kingdom, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Department Neurodegenerative Diseases, Hertie Institute for Clinical Brain Research, Tübingen & Center for Neurology, Tuebingen, Germany, 6German Center for Neurodegenerative Diseases (DZNE), Tuebingen, Germany
Synopsis
Quantification of spontaneous mechanical activities in musculature like fibrillations or fasciculations is of high interest for the assessment of neuro-muscular function in normal and impaired subjects. The diagnostic assessment of neuromuscular disease focuses at specific muscular regions, and the measurement protocol was optimized in order to robustly quantify spontaneous activities in these areas. This work shows preliminary results regarding activity patterns of healthy and diseased subjects.
Introduction
Spontaneous mechanical activities of human resting musculature (SMAM)1 can be assessed by diffusion-weighted imaging (DWI) in MRI. Quantification and detection of these small muscular movements is of high interest for diagnostic assessment in patients with neuromuscular disease (NMD).2 Previous works have shown that SMAMs can be measured in many muscular areas in healthy subjects. However, patients with neuromuscular disorders might show intensified fasciculations or other unintended muscular activities in specific muscular regions, e. g. in the tongue. The ability of the MR sequence to detect spontaneous muscle activity strongly depends on the selected diffusion-sensitive time.3The present study investigates the feasibility of measuring spontaneous activities in specific regions which are important in the assessment of NMD. In addition, the influence of the diffusion sensitivity of the MR sequence on the detectability in patients is evaluated. Preliminary results from four patients are shown.
Methods
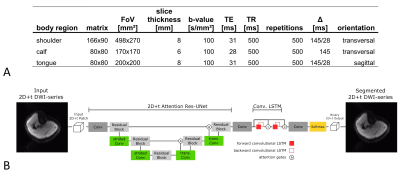
Diffusion-weighted measurements were performed in 6 healthy subjects (age: 30±11 years, gender: 6 male) and four patients with potential neuromuscular disorder (age: 63±9 years, gender: 3 male, 1 female) on a 3T MR system (MAGNETOM Prismafit, Siemens Healthcare, Erlangen, Germany). A prototype diffusion-weighted stimulated-echo EPI sequence was applied for recording spontaneous muscular activities under free-breathing conditions.3,4 Protocol parameters were optimized for the different body regions (Fig. 1A). To enhance patient comfort and reduce overall scan-time for each participant, relevant body regions were selected according to the clinical symptoms, e. g. paresis of lingual musculature, muscle weakness, or motor coordination disorder. Relevant body regions were: 1) calf musculature at the location of maximum diameter (patients: P1, P2, P4); 2) shoulder musculature at the region near to the humeral head (P1, P2, P3); 3) lingual musculature (tongue) centered at the septum (P2, P3). Measurements of the tongue were recorded in sagittal orientation due to better visualization as compared to transversal orientation. Time-series of motion-sensitive single-shot DWI measurements were co-registered before further processing since measurements were conducted under free-breathing conditions. All images were therefore registered by a Local-All-Pass5,6 registration. Spontaneous activities were automatically analyzed by a pre-trained neural network7 with the following building blocks: encoder-decoder structure8,9, residual blocks10, attention-gates11, and prediction smoothing by convolutional long short-term memory (CLSTM)12,13 blocks (Fig. 1B). Results were masked by a user-defined region-of-interest to discard motion-corrupted regions (e.g. tissue compartments directly at the lung). Furthermore, signal dropouts in the tongue musculature due to swallowing were manually removed. Activity was analyzed in terms of the number of SMAM-affected signal dropouts in DWI and event count maps (ECM: summation of activities over repetitions).Results & Discussion
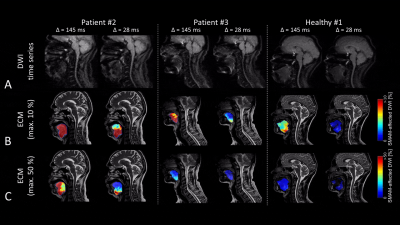
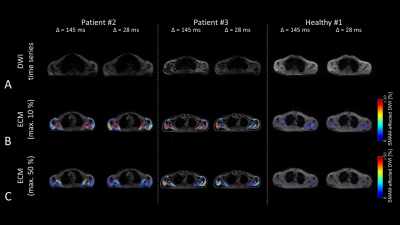
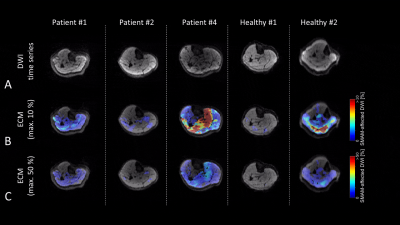
Results of the different muscular regions are given in Fig. 2-4. Image quality at the shoulder regions is reduced for enlarged diffusion sensitivity Δ due to a shortened recovery time. For subjects with a high rate of SMAMs, the sensitivity of the DWI sequence with Δ = 145 ms seemed to be too high, and therefore small differences in the activation pattern cannot be visualized (Fig. 2B, P2). Image quality in the region of the tongue can be highly disturbed by dental treatment which is not uncommon with increased age (Fig. 2, P3). A high rate of SMAM was measured for P2 in the tongue despite an at least partial paresis and linguistic disorder (Fig. 2, P2). Clinically reported fasciculations were in good accordance with the findings in P2 and P3 at the shoulder musculature (Fig. 3B), both revealing high activation patterns. Rather high activation was measured in P4 at the calf musculature (Fig. 4) in contrast to P1, P2 as well as most healthy subjects (P4 suffers from a muscle coordinate disorder). However, high rates of SMAMs can also be measured in some healthy subjects (Fig. 4, Healthy #2). Marked inter-individual differences over different body regions were detected ranging (in an overall percentage of SMAM-affected DWI) in 25±42/13±3/8±9% (calf/shoulder/tongue) for the healthy subgroup and in 47±47/85±21/49±46% for the subgroup with neuromuscular disorders.Conclusion
Quantification of SMAMs in specific muscular regions might present a novel imaging biomarker candidate for neuromuscular/neurodegenerative diseases. Based on our findings, DWI sequences with moderate SMAM-sensitivity should be able to differentiate activation patterns in subjects with a high rate of spontaneous activities. Since healthy subjects can also show a high activation pattern in skeletal musculature, larger studies with patients suffering from neuromuscular/neurodegenerative disease should be set up to investigate potential small differences in the activation patterns.Acknowledgements
This work was supported and funded by the German Research Foundation (DFG) under Grants SCHI 498/11‐1 and YA 28/16‐1.References
[1]: Steidle G,
Schick F. Addressing spontaneous signal voids in repetitive single-shot DWI of
musculature: spatial and temporal patterns in the calves of healthy volunteers
and consideration of unintended muscle activities as underlying mechanism. NMR Biomed 28(7):801-10, 2015.
[2]: Whittaker R,
Porcari P, Braz L, Williams TL, Schofield IS, Blamire A. Functional magnetic
resonance imaging of human motor unit fasciculation in amyotrophic lateral
sclerosis. Annals of Neurology 85(3),
2019.
[3]: Schwartz M,
Steidle G, Martirosian P, Ramos-Murguialday A, Stemmer A, Yang B, Schick F.
Estimation of the Sensitivity Characteristics and Detection Capability of
Diffusion-Weighted MR Sequences in Imaging Spontaneous Mechanical Activity in
Musculature. Proceedings of the Annual Meeting
ISMRM, Honolulu, USA, 2017.
[4]: Schwartz M,
Martirosian P, Küstner T, Steidle G, Feiweier T, Yang B, Schick F. Whole-Body
Mapping of Spontaneous Mechanical Activities in Musculature. Proceedings of the Annual Meeting ISMRM,
Montreal, Canada, 2019.
[5]:
Gilliam C, Küstner T, Blu T. 3D Motion Flow Estimation using Local All-Pass
Filters. Proc. IEEE Int. Symp. Biomed.
Imag. (ISBI), Prague, Czech Republic, 2016.
[6]: Küstner
T, Neumann V, Schwartz M, Würslin C, Martirosian P, Gatidis S, Schwenzer NF,
Schick F, Yang B, Schmidt H. An MR Motion Correction toolbox for registration
and evaluation. Proceedings of the Annual
Meeting ISMRM, Singapore, 2016.
[7]:
Schwartz M, Küstner T, Martirosian P, Machann J, Steidle G, Yang B, Schick F.
Robust Quantification of Spontaneous Muscular Activities by Simultaneous
Interpretation of sEMG Data. Proceedings
of the 36th Annual Scientific Meeting ESMRMB, Rotterdam,
Netherlands, 2019.
[8]:
Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical
Image Segmentation. Medical Image Computing and Computer-Assisted Intervention
(MICCAI), Munich, Germany, 2015.
[9]: Milletari
F, Navab N, Ahmadi SA. V-Net: Fully Convolutional Neural Networks for
Volumetric Medical Image Segmentation. arXiv:1606.04797,
2016.
[10]: He K,
Zhang X, Ren S, Sun J. Deep Residual Learning for Image Recognition. IEEE Conference on Computer Vision and
Pattern Recognition (CVPR), Las Vegas, USA, 2016.
[11]: Oktay
O, Schlemper J, Le Folgoc L, Lee M, Heinrich M, Misawa K, Mori K, McDonagh S,
Hammerla NY, Kainz B, Glocker B, Rueckert D. Attention U-Net: Learning Where to
Look for the Pancreas. Conference on
Medical Imaging with Deep Learning (MIDL), Amsterdam, Netherlands, 2018.
[12]:
Hochreiter S, Schmidhuber J. Long Short-Term Memory. Neural Computation 9(8):1735-80, 1997.
[13]: Shi X, Chen Z, Wang H, Yeung DY, Wong Wk, Woo
Wc. Convolutional LSTM Network: A Machine Learning Approach for Precipitation
Nowcasting. arXiv:1506.04214, 2015.
Figures