2709
Assessment of peripheral muscle deconditioning using 31P-MRS during high intensity ischaemic plantar flexion exercise1Sir Peter Mansfield Imaging Centre, Nottingham, United Kingdom, 2University of Nottingham, Nottingham, United Kingdom, 3Nottingham Digestive Diseases Centre, Nottingham, United Kingdom
Synopsis
Non-invasive assessment of muscle quality is of relevance in chronic disease, where muscle deconditioning is prevalent. To quantify PCr kinetics, localised and non-localised 31P acquisitions were performed during ischemic plantar flexion exercise and recovery. Application of non-localised 31P acquisition resolved motion induced phasing issues and low temporal resolution apparent in 31P ISIS data acquisitions. This allowed quantitation of PCr kinetics in response to high intensity ischemic exercise, successfully providing a surrogate marker of muscle quality in fatigued CD patients. Application of this within-bore exercise protocol to fatigued CD patients and healthy control volunteers will provide insight into pathological fatigue mechanisms.
Introduction
Skeletal muscle is integral to both metabolic and physical function1. Declines in muscle mass and metabolic capacity (deconditioning) are characteristics of ageing and disease progression that collectively manifest as premature fatigue. 31P MRS allows the non-invasive assessment of muscle deconditioning status by quantifying phosphocreatine (PCr) recovery kinetics2 following intense exercise with blood flow occlusion, which is entirely mitochondrial dependent3. Bioenergetic failure within skeletal muscle is a well-established cause of skeletal muscle fatigue4 and may correlate with fatigue perception in chronic disease, such as Crohn’s disease (CD)5. Here, we describe the establishment of a 31P-MRS protocol for use during high intensity ischaemic plantar flexion exercise to quantify PCr depletion and its subsequent recovery kinetics as an index of muscle deconditioning. We aim to investigate this purported premature fatigue in CD patients, who present with clinically significant fatigue perception and reduced skeletal muscle performance relative to healthy control volunteers5.Methods
Acquisition: Data was collected on a 3T Philips Achieva scanner. The participants’ dominant limb was chosen for exercise, Volunteers lay in a supine position on the MRI bed with the knee at approximately 30° with their foot secured into an MRI-compatible plantar flexion ergometer (Trispect, Ergospect, Innsbruck Austria). A 14cm Phillips 31P coil was positioned over the medial gastrocnemius and a blood pressure cuff was placed on the lower thigh. First 1H mDIXON scans were acquired to image the calf. A ~ 12 minute protocol was performed during which 31P data was acquired. Following baseline 31P assessment for ~ 1 minute, the blood pressure cuff was inflated to 250mmHg for 2-minutes to deplete muscle myoglobin concentrations. Volunteers then performed repeated plantar flexion exercise under ischaemia at either a standardised absolute workload, or at 50% of maximum voluntary contraction (MVC), ascertained / performed prior to data acquisition. Volunteers performed 30 contractions per minute, in time with a metronome. Upon exercise cessation, the cuff pressure was released and 31P acquisition continued during exercise recovery. The use of both non-localized pulse-acquire 31P-MR spectra and 31P Image Selected In Vivo Spectroscopy (ISIS) (localising to the medial gastrocnemius) to assess 31P changes during exercise were compared, along with the effects of exercise intensity.Data Analysis: 31P spectra were analysed using jMRUI Beta 6.0, spectra were apodized to 10Hz and zero and first order, phase correction performed. Spectra were frequency aligned and an ER filter applied between 500 -1000Hz. 31P spectra peaks were quantified via the AMARES function. Standardised prior knowledge and starting values were applied from 31P skeletal muscle spectra. Adjustments were made where necessary for inter-individual variations in peak frequency in order to optimise peak fitting. Peak amplitudes were derived for inorganic phosphate (Pi) and PCr and exercise kinetics plotted. The PCr recovery rate constant, k, was computed using a two parameter monoexponential fit to: PCr(t) = PCrinitial+(PCrend – PCrinitial)(1-exp(-k.t)), where t is the time from the start of recovery, PCrinitial and PCrend is the PCr content at the initial and end of recovery phases respectively. PCrend was calculated from the mean end-recovery PCr values and then used in a fit to obtain k and PCrinitial.
Results
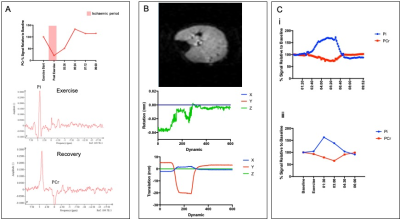
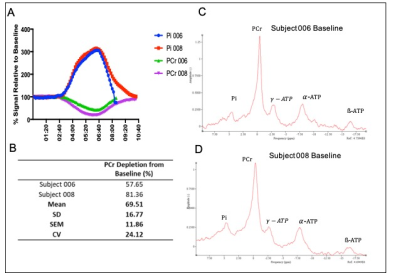
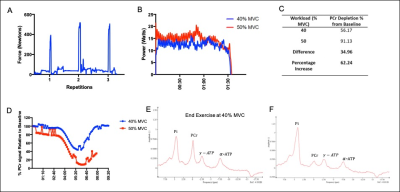
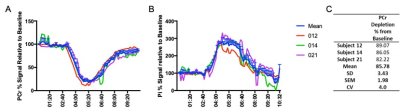
Figure 1 shows 31P ISIS data collected during the ischemic plantar flexion exercise, highlighting the limited temporal resolution and significant phasing issues (Fig 1A) related to motion induced from both the BP cuff inflation and the ischemic exercise protocol (Fig.1B). The advantage a non-localised 31P sequence (Fig1Ci) is clear, overcoming motion issues as the 31P coil moves with the leg. Figure 2 shows inconsistent PCr depletion in two patient volunteers to the same absolute workload (10W) (coefficient variation of PCr depletion 24.12%). Figure 3 shows the effects of using a relative workload, at 40 % and 50 % of maximum voluntary contraction (MVC). Increasing the ischemic exercise intensity from 40 to 50% MVC increased PCr depletion by 62.24% (56.17 vs 91.13%). Figure 4 shows example data for the finalised protocol using 50% MVC during ischaemic plantar flexion exercise, and associated 31P kinetics, mean PCr depletion rate was 85.8 ± 2.0% with a coefficient variation of 4% across three fatigued CD patients. The mean recovery rate constant (k) was 0.57±0.06 min-1.Discussion
Here the development and application of a protocol to study 31P kinetics has been described. It is shown that 31P ISIS has limitations for the study of skeletal muscle PCr recovery kinetics due to its poor temporal resolution (which can be overcome by using a moving average) and sensitivity to motion, induced by both high pressure cuff occlusion and ischemic exercise stimulus. A non-localised 31P measure together with application of a high relative exercise intensity (50 % MVC) during ischaemic plantar flexion exercise is shown to provide a robust measure to study PCr depletion and recovery, and has been applied in participants with quiescent Crohn’s disease. This will allow non-invasive quantitation of muscle metabolic quality in CD to ascertain whether premature performance fatigability reported in CD is driven via peripheral muscle deconditioning.Conclusion
A robust protocol for the assessment of 31P kinetics has been developed which allows extensive depletion of PCr and monitoring of recovery.Acknowledgements
This research is funded by Crohns and Colitis UK (CCUK).References
1.Wolfe R.R. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475-4822.
2. Kent-Braun J.A, McCully K.Kand Chance B. Metabolic effects of training in humans: a 31P-MRS study. Am J Physiol. 1990;69(3):1165-11703.
3. Meyer R.A, Sweeney H.L and Kushmerick M.J. A simple analysis of the “phosphocreatine shuttle”. Am J Physiol. 1984;246(5):C365-774.
4. Sundberg C.W and Fitts R.H. Bioenergetic basis of skjeletal muscle fatigue. Curr Opin Physiol. 2019;10: 118-1275.
5. van Langenberg D.R, Gatta P.D, Warmington S.A, et al. Objectively measured muscle fatigue in Crohn's disease: Correlation with self-reported fatigue and associated factors for clinical application. J Crohns Colitis. 2014;8(2): 137-146.
Figures