2705
31P MRS assessments of mitochondrial dysfunction in patients with peripheral arterial disease undergoing revascularization.1Université de Lyon, INSA-Lyon, Université Claude Bernard Lyon 1, UJM-Saint Etienne, CNRS, Inserm, CREATIS UMR 5220, U1206, Lyon, France, Lyon, France, 2SIemens Healthcare SAS, Saint-Denis, France, 3Wolfson Brain Imaging Center, University of Cambridge, Cambridge, United Kingdom, 4Radiology, Michigan state university, East Lansing, MI, United States
Synopsis
Obliterative arterial disease of the lower limbs is a disease that obstructs lower extremities arteries, resulting in reduced lower limb perfusion and possibly mitochondrial dysfunction. Mitochondrial function of the calf assessed via 31P MRS at moderate and low exercise intensities before and after revascularization and phase contrast angiography of the posterior tibial artery enabled the assessment of vascular and mitochondrial contributions of the patients.
Introduction
Obliterative arterial disease of the lower limbs also known as peripheral arterial occlusive disease (PAOD) is defined as partial or total obstruction of one, or more, lower extremity arteries most often of atherosclerotic origin [1,2]. It is a common pathology whose five-year mortality of a patient with PAOD is about 30%, mainly of cardiovascular origin. In addition, most of the literature suggests that lower extremity mitochondrial function is severely reduced in PAOD. The main objective is to study skeletal muscle mitochondrial function by 31P spectroscopy (τPCr) before and after revascularization in patients with PAOD using an exercise paradigm that approaches the ischemic stress stage and using an alternative exercise paradigm that is well below ischemic stress[3,4].This approach allow insight into the relative contribution of the vascular and mitochondrial components to the degradation of muscle function in PAOD. The overall hypothesis was that slowed τPCr in PAOD is the result of reduced skeletal muscle perfusion. Successful revascularization should be accompanied by an immediate improvement of the τPCr in the absence of mitochondriopathy. On the other hand, in the case of mitochondrial disease, the τPCr should remain altered in early post-revascularization and despite the lifting of the vascular factor.
Methods
Experimental protocolFour patients (male, age=60±6 yrs old, mean±SD) were measured before and after revascularization. Each patient was supine on the MR bed of the scanner, with a surface coil 31P/1H Tx/Rx under the calf muscle.
For dynamic measurements, an MR compatible ergometer was used during isometric plantar flexion. MR scans were performed using a non-localized, MR-FID sequence with saturation bands done with adiabatic pulses[5].
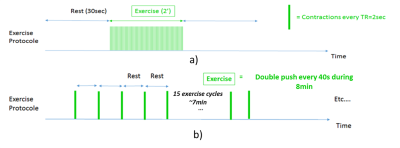
Maximum Voluntary Contraction (MVC) force were performed for 1-2 s. Prior localization was done with a dixon sequence (FA 9deg , TR 4.1ms, TE 1.4ms, matrix size 118×192 , FOV 169×206 ). Thus, resting 31P acquisitions were done with a 90deg FA, TR 30s,10 averages, bandwidth 2500Hz, and saturation bands placed on anterior muscles. Then, 2 dynamic protocols were performed with TR 4s, but with different dynamic resolution times (time between two exercises), see figure 1. Thus, a moderate intensity exercise protocol, expected to approach the ischemic stage as well as a low intensity gated exercise protocol that would be well above the ischemic limit were performed by each subject before and after revascularization. A total of 30 contractions were performed in the moderate protocol at a rate of 0.25 Hz and a total of 15 contractions were performed in the gated protocol at a rate of 0.05 Hz. In addition, resting flow in the posterior tibial artery was assess with ECG gated phased contrast MR angiography.
Data Processing
Data was processed using CSIAPO software[7] for phasing, MATLAB(The MathWorks) and the method QUEST(QUantitation based on QUantum ESTimation)[8]. For the gated protocol, the last 10 contractions cycles were averaged together, resulting in 10 points during the contraction/recovery cycle. In addition, 10 spectra were averaged over the initial rest. The following formula was used to estimate τPCr gated:
τ=-Δt/ln[D/(D+Q)],
where D represents the PCr drop to the steady state (rest PCr–max PCr after steady state is reached), Q is the PCr change in the steady state (max PCr in the steady state–minimum PCr), and t=time between contractions[6]. pH was determined using the frequency difference between PCr and Pi as:
pH=pKa1+log((FreqPi−FreqPCr)−pKam)./pKaM−(FreqPi−FreqPCr),
with pKa1=6.75, pKam=3.27, pKaM=5.69 and FreqPCr and FreqPi the resonant frequencies of PCr and Pi[9].
For blood velocity and flow analyses, velocity was extracted from each time point across the cardiac cycle. Flow was the product of the vessel CSA and velocity averaged across the vessel. Data were compared using paired t-tests to examine differences between exercise protocol and between measurement times with significance at p<0.05.
Results
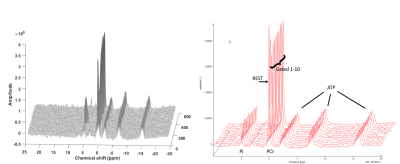
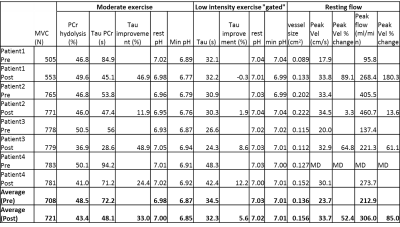
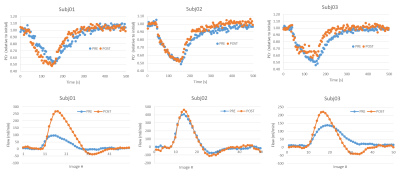
Figure 2 shows a sample stack plot of spectra from the moderate exercise protocol and the low intensity gated protocol; Table 1 reports the MRS results. In general, the PCr recovery time constant was substantially longer for the moderate compared to the gated exercise protocol before surgery (τ=72.2s vs. 34.5s, respectively, p=0.012). Following surgery, there was a 33% improvement of the τPCr for the moderate protocol (p=0.039). However, there was no significant improvement in τPCr for the gated protocol. Blood flow parameters are reported in Table 1. The arterial waveforms are shown in Figure 3. The waveforms in 3 cases are characterized as monophasic. Following revascularization, there was an apparent increase in peak blood velocity that approached significance. This was particularly the case for Subj01 and Subj03 who adopted a biphasic arterial waveform following revascularization.Discussion
The results primarily indicate that blood flow is a large determinant of the measured PCr time constant under standard exercise conditions. This is demonstrated both when comparing the moderate to the low intensity protocol and when examining the moderate exercise PCr time constant post-surgery. These results encourage the quantification of blood flow when assessing mitochondrial function in conditions of compromised flow. Alternatively, a “gated” low intensity protocol may be utilized to independently assess mitochondrial function in conditions of compromised blood flow, oxygen delivery, or oxygen consumption.Acknowledgements
This work has been supported by Siemens Healthineers and the LABEX PRIMES (ANR-11-LABX-0063), program ”Investissements d’Avenir” (ANR-11-IDEX-0007) and carried out within the framework of France Life Imaging (ANR-11-INBS-0006).References
1. Eric P Brass, William R Hiatt, and Simon Green. “Skeletal muscle metabolic changes in peripheral arterial disease contribute to exercise intolerance: a point-counterpoint discussion”. In: Vascular medicine 9.4 (2004), pp. 293–301
2. Eric P Brass and William R Hiatt. “Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease”. In: Vascular Medicine 5.1 (2000), pp. 55–59
3. David C Isbell, Stuart S Berr, Alicia Y Toledano, et al. “Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease”. In: Journal of the American College of Cardiology 47.11 (2006), pp. 2289–2295
4. PIPINOS, Iraklis I., SHEPARD, Alexander D., ANAGNOSTOPOULOS, Petros V., et al. Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. Journal of vascular surgery, 2000, vol. 31, no 5, p. 944-952
5. LUO, Y., DE GRAAF, R. A., DELABARRE, L., et al. BISTRO: an outer‐volume suppression method that tolerates RF field inhomogeneity. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 2001, vol. 45, no 6, p. 1095-1102
6. Jill M Slade, Theodore F Towse, Mark C DeLano, Robert W Wiseman, and Ronald A Meyer. “A gated 31P NMR method for the estimation of phosphocreatine recovery time and contractile ATP cost in human muscle”. In: NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo 19.5 (2006), pp. 573–580
7. Yann Le Fur, François Nicoli, Maxime Guye, et al. “Grid-free interactive and automated data processing for MR chemical shift imaging data”. In: Magnetic Resonance Materials in Physics, Biology and Medicine 23.1 (2010), pp. 23–30
8. Helene Ratiney, Mark J Albers, Herald Rabeson, and John Kurhanewicz. “Semi-parametric time-domain quantification of HR-MAS data from prostate tissue”. In: NMR in biomedicine 23.10 (2010), pp. 1146–1157
9. LANZA, Ian R., BHAGRA, Sumit, NAIR, K. Sreekumaran, et al. Measurement of human skeletal muscle oxidative capacity by 31P‐MR spectroscopy: a cross‐validation with in vitro measurements. Journal of Magnetic Resonance Imaging, 2011, vol. 34, no 5, p. 1143-1150
Figures