2701
Diffusion tensor imaging of calf muscle damage in peripheral arterial disease
Jianli Wang1, Jonathan Stavres2, Christopher T Sica1, Cheryl Blaha2, Michael Herr2, Samuel Pai2, Aimee Cauffman2, Jeffrey Vesek3, Qing X Yang1,4, and Lawrence I Sinoway2
1Radiology, Penn State College of Medicine, Hershey, PA, United States, 2Heart & Vascular Institute, Penn State College of Medicine, Hershey, PA, United States, 3Molecular Biology, Penn State College of Medicine, Hershey, PA, United States, 4Neurosurgery, Penn State College of Medicine, Hershey, PA, United States
1Radiology, Penn State College of Medicine, Hershey, PA, United States, 2Heart & Vascular Institute, Penn State College of Medicine, Hershey, PA, United States, 3Molecular Biology, Penn State College of Medicine, Hershey, PA, United States, 4Neurosurgery, Penn State College of Medicine, Hershey, PA, United States
Synopsis
Peripheral arterial disease (PAD) is a systemic atherosclerotic vascular disease characterized by impaired skeletal muscle perfusion and mitochondrial respiration. Over time, persistent exposure to muscle ischemia eventually leads to muscle atrophy, myopathy, and mitochondrial dysfunction in PAD patients. In this study we investigated the utilization of diffusion tensor imaging to characterize the pathophysiological changes of calf muscles before and after graded plantar flexion exercise. At rest, the clinically worse leg of the PAD subjects had higher diffusivity in the calf muscles. Significant greater increases of diffusivity in the post-exercise muscle of PADs than the HCs suggest acute exercise-related muscle damage.
Introduction
Peripheral arterial disease (PAD) is a systemic atherosclerotic vascular disease that affects over 200 million people worldwide [1,2]. Symptoms most often manifest during light to moderate-intensity exercise. It characterized by impaired skeletal muscle perfusion and mitochondrial respiration. The pathophysiology in the muscle of PAD is complicated. Over time, persistent exposure to muscle ischemia eventually leads to muscle atrophy [3], myopathy [4-6], and mitochondrial dysfunction [7,8] in PAD patients. Since skeletal muscle fibers are highly oriented, in this study we investigated the utilization of diffusion tensor imaging (DTI) to characterize the pathophysiological changes of skeletal muscles in PAD before and after light to moderate-intensity exercise.Methods
8 PADs [ankle brachial index (ABI) < 0.9 in one leg] and 7 age/sex-matched healthy controls (HC) participated in the study (Table 1). Most subjects completed two visits for the study of both legs, except 2 PAD and 2 HC subjects completed one visit for one leg. Each visit consisted of 2 runs of unilateral graded plantar flexion exercise using a custom built non-ferromagnetic plantar flexion device inside a Siemens Prisma Fit 3T MRI scanner: one run of exercise in the imaged leg (same leg exercise, SLE), and a second run in the opposite leg (opposite leg exercise, OLE). The exercise paradigm began at a 2 kg workload (30 contractions/minute) and increased by 2 kg every 2 minutes until fatigue, or until the completion of 10 kg. This exercise protocol was designed to maximize the period of pre-symptomatic exercise PAD patients while also eliciting a typical cardiovascular response to exercise in HCs.During each visit, DTI of one leg's calf muscles was conducted before and after both SLE and OLE with a 15-channel transmit-receive coil and a spin-echo echo-planar DTI sequence. The thickest part of the calf that labeled with a small cross-shaped fiducial marker was put in the center of the coil. The scan parameters were TR/TE/FA = 28 s/70.40 ms/90°, 32 4-mm-thick transverse slices, image resolution = 1.8 mm × 1.8 mm, multi-band acceleration factor = 2, b-value = 0, 200, 400, 600 s/mm2, 30 angular diffusion gradient directions, four b0 images interleaved with the diffusion-weighted images.
The DTI data was processed with Diffusion Toolkit 0.6.4.1 and TrackVis. For quality control of the motion artifact, the four b0 images acquired from the beginning to the end of each DTI data acquisition were realigned using SPM12 with a threshold of movement < 1 mm and rotation < 1º. The majority of medial gastrocnemius (MG) and tibialis anterior muscle (TA) at the level of the fiducial marker were manually labeled as the seed for fiber-tracking of the muscle fibers for each subject. Average diffusion parameters [mean diffusivity (MD), fractional anisotropy (FA), and eigenvalues (λ1-3)] of the muscle fiber bundle was recorded and compared across pre-post exercise and between two legs with paired t-test, and between PAD and HC groups with ANOVA.
Results
At rest, there were no significant differences in MD, FA, or λ1-3 for the MG or TA between groups (p>0.09). However in the PAD group, the leg with higher ABI had significant higher MD, λ1-2 in the MG and TA (paired t-test, n=6, p≤0.04, Figures 1&2). After SLE, in both PADs and HCs, there were significant diffusivity increases in the MG, but not in the TA (paired t-test, p≤0.03); while the diffusivity increases in the PADs were significantly higher than the HCs (ANOVA, p≤0.03, Figure 3a&b). After OLE, there was no significant diffusivity change in the MG of either group.Discussion and Conclusion
At rest, the clinically worse leg of the PAD subjects had higher diffusivity in the calf muscles. After SLE, significant diffusivity changes were observed in the MG of HCs, the active muscle involved in plantar flexion exercise, but not in the inactive TA. This indicates that DTI is sensitive to characterize exercise-related physiological changes in the muscles. Significant greater increases of diffusivity in the post-exercise muscle of PADs than the HCs suggest acute exercise-related muscle damage.Acknowledgements
This project was supported by NIH P01 HL134609 and UL1 TR002014.References
1. Benjamin EJ, et al. Circulation. 2017; 135(10):e146-e603. 2. Shu J & Santulli G. Atherosclerosis. 2017;275:379-81. 3. Regensteiner JG, et al. Circulation. 1993; 87(2):413-21. 4. Ha DM, et al. J Transl Med. 2016;14:39. 5. Makitie J & Teravainen H. Arch Pathol Lab Med. 1977;101(12):658-63. 6. Mitchell RG, et al. Vasc Med. 2007;12(4):285-90. 7. AlGhatrif M, et al. J Am Heart Assoc. 2017;6(9). 8. Pipinos II, et al. Vasc Endovascular Surg. 2007;41(6):481-9.Figures
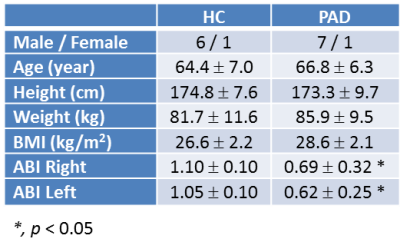
Table 1. demographics and clinical measurements
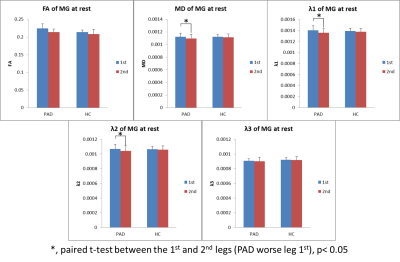
Figure 1. At rest, significant higher diffusivity in the MG of worse legs in PADs.
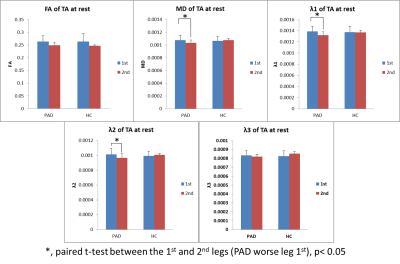
Figure 2. At rest, significant higher diffusivity in the TA of worse legs in PADs.
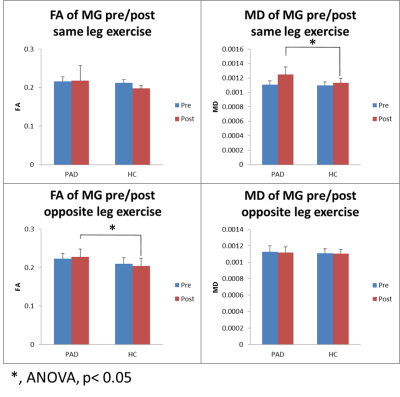
Figure 3a. PADs had significantly higher diffusivity in MG than the HCs post same-leg exercise.
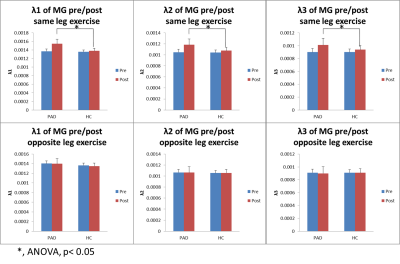
Figure 3b. PADs had significantly higher diffusivity in MG than the HCs post same-leg exercise.