2695
Myoelectric Triggered Diffusion-Weighted Imaging for Muscle Contraction Analysis
Martin Schwartz1,2, Petros Martirosian1, Günter Steidle1, Michael Erb3, Bin Yang2, and Fritz Schick1
1Section on Experimental Radiology, University Hospital of Tuebingen, Tuebingen, Germany, 2Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany, 3Biomedical Magnetic Resonance, University Hospital of Tuebingen, Tuebingen, Germany
1Section on Experimental Radiology, University Hospital of Tuebingen, Tuebingen, Germany, 2Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany, 3Biomedical Magnetic Resonance, University Hospital of Tuebingen, Tuebingen, Germany
Synopsis
Retrospective measurements of muscular contraction in diffusion-weighted imaging are inherently asynchronous leading to an unknown time point of acquisition during the muscular motion. Therefore, prospective imaging is investigated based on surface electromyography signals derived during the measurement. Fast and more robust real-time activity detection is achieved by a neural network. Imaging during active muscular contraction can be prevented by analysis of the muscular state; however, sampling at different time points of the muscular contractions is also possible.
Introduction
Diffusion-weighted magnetic resonance imaging (DW-MRI) is sensitive to record incoherent muscular motion like small spontaneous mechanical activities in musculature (SMAM)1. Simultaneous recordings of DWI and surface electromyography (sEMG) have shown high correlation between both modalities.2 Furthermore, a high variation of the visualization capability was revealed, depending on MR sequence parameters.3 Therefore, improved detection and visualization of muscular motion was investigated by sEMG-triggered MR-DWI in previous studies4,5. A tradeoff between average systemic delay between onset of sEMG activity and MR-DWI4, and computational complexity has to be considered5. In this work, an improved system combining a short systemic delay with a highly adaptable noise-robust detection by neural network architectures is investigated to overcome limitations of previous works4,5. Moreover, preliminary results regarding the influence of the trigger time delay on the visualization of spontaneous activities and active muscular contractions are presented.Methods
Model-based event detection and MR system triggering is achieved by adaption of a sequence controller5 for fast signal processing. The system is depicted in Fig. 1. Sequence Controller: The sEMG signal is sampled with fs = 1 kHz and processed galvanically decoupled on a microcontroller system (ARM Cortex-M3). For pre-processing the sEMG signal is filtered (fband-pass=20-500 Hz and fnotch=45-55 Hz) and rectified before it is sent to the host system for storage and visualization and to the detection unit by serial communication. The sequence controller triggers the MR system by a fiber optical connection after receiving information from the detection unit. A flexible delay between sEMG onset and start of DWI measurement can be set to record motion in different states. The acquisition scheme is illustrated in Fig. 2. Detection unit: A single-board computer (Raspberry Pi Foundation, Cambridge, UK) is utilized for sEMG signal classification. For real-time detection, the inference time is reduced by a Coral Accelerator with an Edge Tensor Processing Unit (TPU) (Google LLC, Mountain View, CA, USA) and quantization-aware training6. Therefore, the neural network architecture is restricted to layer types which are supported by the accelerator device: Multiple layers of strided 1D convolution layers (encoder structure) with batch-normalization7 and rectified linear unit (ReLU) activation was utilized with a dense layer as output (softmax activation). Classification output is: "sEMG activity"/"no sEMG activity" (corresponding to a "trigger out" or "no trigger out" to the sequence controller). Training Data: The training procedure is outlined in Fig. 3. sEMG signals for training were acquired during simultaneous DW-MRI with an MR-compatible amplifier (BrainAmp ExG MR, Brain Products GmbH, Gilching, Germany) from 10 healthy subjects (age: 34±13 years, gender: 8m/2f) from previous work2. sEMG signals were semi-automatically annotated.8 For simulation of a sEMG stream, a pre-trained signal generator9 (generative adversarial network10,11 based on long short-term memory cells12,13) was utilized to generate sEMG samples which are superimposed on resting sEMG signals recorded inside the MR system. For a more robust detection, data was augmented by additive white Gaussian noise and harmonic distortions (sinusoidal signals from 1-500 Hz). After offline training, the trained model (TensorFlow Lite, Google LLC) is transferred to the detection unit. System evaluation: sEMG signals were derived from the skeletal musculature by MR-compatible Ag/AgCL electrodes (BrainProducts GmbH, Gilching, Germany) during MR-DWI (3T MAGNETOM Prismafit, Siemens Healthcare, Erlangen, Germany) measurements with a 15 ch. Tx/Rx knee coil from 3 healthy subjects (age: 42±21, gender: male) with following parameterization: TE: 36 ms, b-value: 100 s/mm², 6/8 readout, matrix: 64-80x64-80, no fat-suppression, spin echo or stimulated echo excitation (diffusion-sensitive time: Δ = 145 ms).Results & Discussion
An average inference time on the TPU of 0.4 ms was measured with a test-accuracy of 71.7 % on noisy data. Time between sEMG onset and MR trigger output was 5.4±2.8 ms in average leading to an overall minimum systemic delay of 12.6±4.5 ms due to the necessary trigger pulse length of around 10 ms which is much lower or comparable to previous works4,5. Non-triggered MR-DWI, MR-DWI triggered by spontaneous activity and MR-DWI triggered by active movement are exemplarily depicted in Fig. 4. Clear differences are visible in the near-surface region of the sEMG electrode. Recording of active movements can be prevented by MR acquisition after muscular contraction (prolonged trigger time delay). Fig. 5 shows the influence of the trigger time delay on spontaneous activities. It is shown that with a larger trigger time delay the contraction patterns of spontaneous activities are almost fully relaxed after 175 ms.Conclusion
Robust sEMG activity detection enables studies of small muscular movements or contraction pattern analysis by triggered MR-DWI measurements. Further work should investigate systematically the influence of the neural network architecture, e. g. sequence length and types of layers (dilated temporal convolutional networks), on the detection capability and robustness regarding signal distortions as well as the effect of the trigger time delay on the muscular contraction pattern. Furthermore, the real-time classification can improve the quantitative assessment of spontaneous activities by discarding time periods of active movement14.Acknowledgements
This work was supported and funded by the German Research Foundation (DFG) under Grants SCHI 498/11‐1 and YA 28/16‐1. We thank Feiweier, T., Siemens Healthcare, Erlangen, Germany for his valuable technical support on this project.References
[1]: Steidle G, Schick F. Addressing spontaneous signal voids in repetitive single-shot DWI of musculature: spatial and temporal patterns in the calves of healthy volunteers and consideration of unintended muscle activities as underlying mechanism. NMR Biomed 28(7):801-10, 2015.[2]: Schwartz M, Steidle G, Martirosian P, Ramos-Murguialday A, Preißl H, Stemmer A, Yang B, Schick F. Spontaneous Mechanical and Electrical Activities of Human Calf Musculature at Rest Assessed by Repetitive Single-Shot Diffusion-Weighted MRI and Simultaneous Surface-Electromyography. Magn Reson Med 79(5):2784-94, 2018.
[3]: Schwartz M, Steidle G, Martirosian P, Ramos-Murguialday A, Stemmer A, Yang B, Schick F. Estimation of the Sensitivity Characteristics and Detection Capability of Diffusion-Weighted MR Sequences in Imaging Spontaneous Mechanical Activity in Musculature. Proc. ISMRM, Honolulu, USA, 2017.
[4]: Schwartz M, Martirosian P, Steidle G, Erb M, Yang B, Schick F. Feasibility of real-time surface electromyography-triggered diffusion-weighted imaging for prospective imaging of spontaneous unintentional focal muscular motion in human calf musculature. Proc. ESMRMB, Barcelona, Spain, 2017.
[5]: Schwartz M, Martirosian P, Yang B, Schick F. A Surface Electromyography-Driven Magnetic Resonance Sequence Controller for Real-Time Myoelectric Triggered Imaging. Proc. IEEE EMBC, Honolulu, HI, USA, 2018.
[6]: Jacob B, Kligys S, Chen B, Zhu M, Tang M, Howard A, Adam H, Kalenichenko D. Quantization and Training of Neural Networks for Efficient Integer-Arithmetic-Only Inference. arXiv:1712.05877, 2017.
[7]: Ioffe S, Szegedy C. Batch Normalization: Accelerating Deep Network Training by Reducing Internal Covariate Shift. arXiv:1502.03167, 2015.
[8]: Schwartz M, Steidle G, Martirosian P, Ramos-Murguialday A, Yang B, Schick F. Spontaneous activity in sEMG in relation to signal voids in repetitive single-shot DW-MRI of the human lower leg: Comparison of measurements under varying conditions. Proc. ESMRMB, Vienna, Austria, 2016.
[9]: Schwartz M, Reuter M, Yang B, Schick F. Monte-Carlo Analysis for Quality Estimation of Gradient Correction Algorithms in Simultaneous Surface EMG-MRI Measurements using Signal Synthesis and Class Probability. Proc. IEEE EMBC, Berlin, Germany, 2019.
[10]: Goodfellow I, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S,Courville A, Bengio Y. Generative Adversarial Nets. NeurIPS, 2014.
[11]: Mirza M, Osindero S. Conditional Generative Adversarial Nets. arXiv:1411.1784, 2014.
[12]: Hochreiter S, Schmidhuber J. Long Short-Term Memory. Neural Computation 9(8):1735-80, 1997.
[13]: Graves S. Generating Sequences With Recurrent Neural Networks. arXiv:1308.0850, 2013.
[14]: Schwartz M, Küstner T, Martirosian P, Machann J, Steidle G, Yang B, Schick F. Robust Quantification of Spontaneous Muscular Activities by Simultaneous Interpretation of sEMG Data. Proc. ESMRMB, Rotterdam, Netherlands, 2019.
Figures
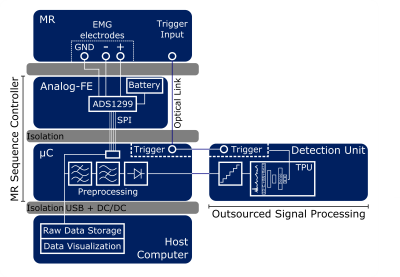
Figure 1: System architecture for myoelectric triggered diffusion-weighted
imaging. MR sequence controller samples the sEMG signal and sends it to the
host computer as well as to the detection unit after signal pre-processing
(band-pass filtering, notch filtering, rectifying). A tensor processing unit is
utilized for applying neural network architectures for signal classification
and activity detection. Positive classification results in a trigger signal for
the MR system.
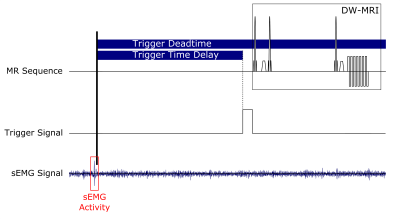
Figure
2: Scheme of DW-MRI sequence triggering. A detected sEMG activity leads to
an MR trigger signal which can be delayed by the MR sequence controller to 1)
enable different sampling time points within the muscular contraction or 2)
prevent imaging during motion contraction (prolonged delay). To prevent
false detections during the running sequence, the MR sequence controller
disable the activity detection within a certain period (trigger deadtime).
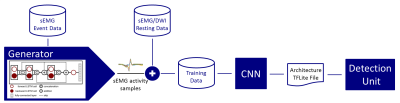
Figure
3: A signal generator is trained on sEMG activities to synthesize short
sEMG samples which are superimposed on resting sEMG to generate an sEMG
training data stream. A convolutional neural network is trained offline for
signal classification/activity detection. Afterwards, the pre-trained model is
transferred on the detection unit for online operation.
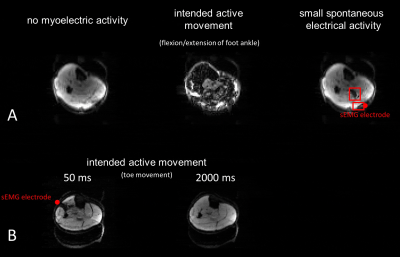
Figure
4: A:
Three different muscular states: resting, intended active movement and small
spontaneous activity are recorded with a diffusion-weighted spin-echo sequence.
Clear differences are visible between all three images. Small spontaneous
activities can only be recorded in near-surface areas around the sEMG
electrode. B: Active movement of the
m. extensor digitorum longus with small trigger time delay and a prolonged
delay (stimulated-echo sequence) is shown. Movement in the prolonged delay is
no longer visible.
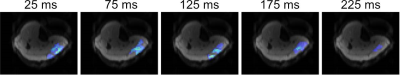
Figure 5: Event
count maps of 40 triggered MR-DWI (spin-echo) with different trigger time delay
were recorded. Trigger time delay was varied from 25-225 ms. A sEMG
electrode was placed on the m. gastrocnemius medialis. More activities were
recorded with a delay period of 25-125 ms in contrast to a reduced number
of activities by using a prolonged delay time due to the muscular relaxation.